ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Airway Stenting
Patient Selection
The purpose of airway stenting is to relieve airway obstruction caused by strictures not suitable for resection and reconstruction. Historical attempts at airway stenting began in the 19th century, and tracheostomy tubes and Montgomery tracheal T tubes have been extensively used to palliate benign and malignant strictures [1]. More recently, a variety of endoscopic techniques have been developed to manage benign and malignant lesions involving the central airway: mechanical debridement, laser resection and vaporization, photodynamic therapy, and endobronchial brachytherapy. Tracheo-bronchial stenting has been extensively performed to palliate dyspnea caused by extrinsic compression, intraluminal disease, and loss of cartilaginous support. Although surgical correction is always preferred when technically feasible and when the clinical status of the patient permits, stenting provides a reliable alternative in selected cases and helps to improve quality of life in non-surgical patients. The airway stenting armamentarium received enormous benefit from the experience gained with intraluminal devices designed for the biliary system, urinary tract, esophagus, and blood vessels. Modern stents allow translaryngeal air flow providing for a humidified airway and preservation of the voice without the need for tracheostomy in most cases.
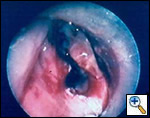
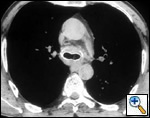
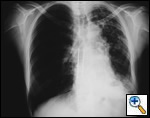
A number of indications for endoscopic treatment have been reported (Table 1) but the most frequent is lung cancer involving the airway proximal to the lobar orifices. In most patients with unresectable tumors only palliation is required [2,3] and sometimes urgent stenting during chemo-radiotherapy may be demanded; however, in selected patients a viable airway may be important as a bridge to surgery [4,5]. Patients with primary airway tumors (Figure 1) may receive benefit from endoluminal stenting if surgery is not indicated. Other tumors that occur adjacent to the airway may produce obstruction by direct invasion or extrinsic compression, including esophageal cancer possibly accompanied by tracheo-esophageal fistula (Figure 2), thyroid cancer, and other head and neck tumors, and may be successfully palliated with endoluminal treatments combined with stent placement [6].
Table I
- Lung cancer
- Primary airway tumors
- Oesophageal cancer
- Thyroid cancer
- Head and Neck tumours
- Metastases
- Postintubation and idiopatic benign tracheal stenosis
- Inflammatory lesions
- Tracheobronchial malacia
- Vascular compression
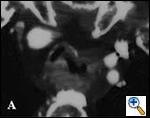
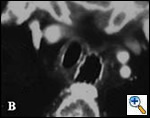
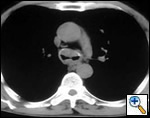
Patients with postintubation tracheal stenosis are occasionally good candidates for airway dilation and stenting, but in most of these patients surgical resection and reconstruction should be performed [7,8]. In patients who are candidates for resection and reconstruction only temporary endoscopic palliation should be considered in preparation for surgery to allow adequate stabilization of the inflammatory lesion [9]. Tracheobronchial stenting should be advocated only for patients with an extremely high surgical risk (always consider that tracheal resection is a neck operation with a relatively low surgical insult), for patients refusing surgery, or for patients with a long stenosis that is not amenable to surgical correction. Some rare benign conditions are appropriate for stenting, including anastomotic stenosis after tracheal and bronchial sleeve resection, inflammatory or infectious conditions causing airway stenosis, vascular compression, and tracheobronchial malacia. Patients undergoing lung transplantation might also benefit from endobronchial stenting if airway complications such as bronchial stenosis or anastomotic dehiscence occur (Figure 3).
 |
 |
| Figure 3: A) Partial bronchial dehiscence on the mediastinal side of the anastomosis after lung transplantation; B) A Dumon stent was placed to allow “protected” airway healing. | |
Operative Steps
Anesthesia
Patients undergoing flexible bronchoscopy for evaluation of their airway problems are routinely managed with local anesthesia and sedation. In such patients, however, general anesthesia and rigid bronchoscopy should be immediately available if required, especially when a critical obstruction of the central airway is present.
 |
| Figure 4: Laryngeal syringe used for laryngeal and upper airway local anesthesia |
Rigid bronchoscopy requires general anesthesia but muscle paralysis is generally not indicated. In most patients ventilation can be managed through the accessory port of the rigid bronchoscope; occasionally a jet ventilation catheter may be inserted, but it usually adds complexity without evident benefit. Generous topical anesthetic should be administered, starting with its instillation in the upper airway through a laryngeal syringe (Figure 4) before inserting the rigid bronchoscope. Additional topical anesthetic is administered during the procedure, in particular before using laser, prior to dilation, and before placing stents, to inhibit the cough reflex. General anesthesia should be induced with Fentanyl (2.5 µg/kg) and Propofol (2.0 mg/kg) and maintained with Propofol (7 – 8 mg/kg/h reduced to 5 – 6 mg/kg/h after 15 minutes); if necessary, before placing stents additional Fentanyl (2 µg/kg) and Propofol (0.5 mg/kg) is administered.
Bronchoscopic Equipment and Operative Technique
It is of paramount importance to work with a dedicated team of anesthesiologists and nurses familiar with the endoscopic maneuvers and the equipment required; this will facilitate the procedure especially in critical situations. Many different endoscopic techniques for palliation of airway obstruction have been described; they should all be considered along with the surgical options when assessing a patient with airway obstruction. In these situations bronchoscopy is essential in the evaluation process and CT virtual bronchoscopy should not be used as a substitute. Bronchoscopy in the critical patient should be performed only by an expert bronchoscopist; it allows the surgeon to define the extent, severity, and complexity of the stenosis. It also permits assessment of potential treatment modalities and directs bronchoscopic intervention. It should be performed by an endoscopist familiar both with interventional bronchology and the potential surgical options.
Both the flexible and rigid bronchoscopes should be available even if some endoscopists tend to favor either the former or the latter. Flexible fiberoptic bronchoscopy allows evaluation and diagnosis under local anesthesia as well as placement of expandable stents with the patient awake. However, rigid bronchoscopy provides a much wider spectrum of interventions; it has the disadvantage of requiring general anesthesia but allows one to quickly achieve a patent distal airway and adequately ventilate the patient, preserving oxygenation in critical situations. During rigid bronchoscopy therapeutic maneuvers can be immediately performed, such as vaporizing endoluminal lesions with laser and placing silicone or expandable metal stents. In case assessment and subsequent treatment of lesions located in the distal airway is required, the flexible bronchoscope can be inserted through the rigid barrel and advanced peripherally. It should always be stressed that even if fiberoptic bronchoscopy is planned as the first step, in critically ill patients it should be performed in the operating room, ready to proceed with the rigid bronchoscope if the clinical and anatomic situation requires it.
Flexible bronchoscopy is performed using an adult bronchoscope with a large working channel (2.8 to 3.2 mm) to allow adequate suction and delivery of laser probes, balloon dilators, and stent delivery devices. Larger stent delivery catheters should be introduced under fluoroscopy after placing external radiopaque markers.
Rigid bronchoscopy allows better and safer management of critical obstructions. We prefer the universal Dumon–Harrel rigid bronchoscope (EFER, La Ciotat, France) that comes in a variety of sizes and is designed with a multiport head allowing ventilation, suction with multiple catheters, and insertion of the laser probe and the telescope.
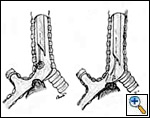 |
| Figure 5: A) Mechanical debridement and B) laser vaporization of an endobronchial neoplastic lesion |
The rigid bronchoscope is introduced under direct vision and is advanced until the obstruction is visualized. The use of telescopes connected to a video system greatly facilitates the procedure, also allowing image magnification. Lesions growing within the airway may be mechanically debrided or vaporized by laser (Figure 5). Extrinsic compressions should be gently dilated with the tip of the bronchoscope until the barrel can be advanced distally to gain a patent lumen and ventilate the patient. The use of rigid bronchoscopes that sequentially increase in diameter may facilitate this maneuver. Postintubation tracheal stenoses should be radially incised in three or four points before advancing the rigid bronchoscope to dilate it. Benign stenoses at the level of the main bronchi (after sleeve lobectomy, transplantation, radiotherapy, tuberculous infection) may pose different problems. The first dilation attempt is sometimes difficult if the stenosis is extremely tight and rigid, providing a large discrepancy between the diameter of the lumen and the caliber of the bronchoscope. In such situations balloon dilation is performed before forcing the rigid scope through the stenosis. In some cases we have successfully used old fashioned metal esophageal Souttar dilators that were rescued from the historical armamentarium of our endoscopy unit (Figure 6). If the tumor is located at the level of the carina and involves the distal trachea and both mainstem bronchi it is extremely important to quickly gain a viable airway and ventilate the patient. When the airway is patent and tumor extension has been carefully evaluated, a Y stent can be placed (Figure 7). Once an adequate airway caliber has been obtained the patency and stability of the lumen should be considered and any tendency for airway collapse requires stent placement. Stenting is also considered to prolong patency if an obstructing tumor is growing inside the airway (Figure 8).
Choice of Stent
Once an adequate caliber of the airway has been obtained stenting may be required for two reasons: 1) the airway tends to collapse or 2) to prolong the period of patency in case of malignant involvement. The final step is the assessment of the type and size of the stent to be placed. There are a number of stents currently available (Table 2) and each one shows advantages and disadvantages. Basically there are two groups of stents: silicone and metal stents. A selection of each category should be always available to optimize treatment and results.
Table II |
|||
| Stent Type | Manufacturer | Construction | Sizes (mm) |
| Dumon | Novatech | Molded silicon rubber | 9 x 20 – 18 x 70 + Y |
| Hood | Hood Corp. | Molded silicon rubber | 6 x 13 – 18 x 70 + Y |
| Wallstent | Boston Scientific | Woven cobalt/chrome alloy monofilament coated with silicone | 8 x 20 – 24 x 60 |
| Polyflex | Rush Inc. | Polyester mesh covered with silicone | 6 x 20 – 22 x 80 |
| Ultraflex | Boston Scientific | Single strand woven nitilol With/without silicone coating | 8 x 20 – 20 x 80 |
| Dynamic | Rush Inc. | Silicone with anterolateal steel struts | 13, 15, 17 (trachea) |
 |
| Figure 9: Dumon stents of different sizes and lengths |
The primary advantage of silicone stents is that they are easily adjustable and removable, and can be repositioned and changed as many times as required; with these stents there is no ingrowth and no reaction of the airway mucosa. The Dumon silicone stents (Novatech, Plan de Grasse, France) were specifically designed for the airway (Figure 9) [10]. The cylindrical form provides a vault effect by which compressive forces are evenly distributed. Flexibility facilitates placement and removal, improves tolerance, and tends to preserve clearance of secretions. The studs on the outer surface of the stent prevent migration and reduce mucosal ischemia by limiting contact with the airway wall. A wide range of sizes and diameters are available (from 9 to 18 mm in external diameter and from 20 to 60 mm in length) so that stenting can be limited to the stenotic zone, encompassing only 0.5 cm above and below it; in fact, minimizing the length of the stent is a key factor in maintaining clearance of secretions and enhancing tolerance. The rims of each stent are polished to remove burrs and to reduce the risk of granuloma formation. Radiopaque Dumon stents are also available to improve visualization on chest x-ray. Obvious disadvantages of this type of stent are the need for rigid bronchoscopy for placement, along with some potential difficulties during deployment. A legitimate criticism is the smaller inner diameter due to the thicker wall of the stent and the potential for dislodgement and distortion. The loss of mucociliary clearance has a lower impact on secretion retention with the new generation stents. The Montgomery T tube (Figure 10) is still a useful stent and should be always considered for patients with a tracheostomy and a working larynx. Silicone Hood stents (Pembrooke, MA, USA) (Figure 11) have almost the same characteristics as the Dumon stents but have no studs on the outer surface.
 |
 |
| Figure 10: Montgomery T-tube | Figure 11: Hood stents of different sizes |
In contrast, expandable metal stents can be easily delivered using a flexible bronchoscope under local anesthesia using fluoroscopy. These stents are extremely stable and migration is virtually impossible. The most recent generation of expandable stents (Wallstent and Ultraflex) conforms much better to the anatomy of the airway. Expandable stents may be covered (silicone rubber or polyurethane) or uncovered. Uncovered stents (Figure 12) are eventually incorporated within the airway wall with neoepithelization and resumption of mucociliary clearance. Covered stents (Figure 13) should be used in patients with malignant strictures when the tumor tends to grow within the airway. Uncovered stents allow also ventilation of lobar bronchi through the interstices of the metal mesh in case the airway needs to be stented above and below these orifices. However, these stents show some disadvantages: they are permanent since removal is extremely difficult, if not impossible; adjustment is difficult; fluoroscopy is required during placement; and granulations tend to grow at the level of the uncovered edges. If uncovered expandable stents are used to support neoplastic stenoses, they may erode the wall of the airway and the tumor may grow through the mesh. Last but not least, they are much more expensive than silicone stents. At our institution, the only indication for uncovered stents is airway malacia.
 |
 |
 |
| Figure 12: Uncovered Ultraflex stent | Figure 13: Covered Ultraflex stent | Figure 14: Polyflex expandable Rush stents |
The Polyflex expandable stents (Figure 14) are made of a polyester mesh coated with silicone; they are self expandable and constrained within a delivery catheter. These stents don’t have uncovered edges and their potential advantages and disadvantages place them somewhere between the silicon and the expandable metal stents.
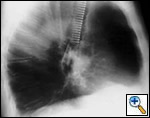
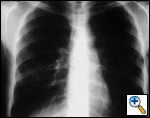
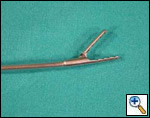
Freitag and colleagues extensively described the use of the Dynamic stent (Figures 15, 16) in patients with benign and malignant airway obstructions. This stent shows the potential advantage of having a flexible “membranous wall” able to squeeze down during coughing, facilitating mucous clearance. Placement of this stent is potentially more complicated but dedicated delivery grasping forceps have been designed to facilitate it (Figure 17). Results are promising for management of distal tracheal, carinal, and mainstem bronchial obstructions.
 |
 |
 |
| Figure 15: Dynamic Freitag stent | Figure 16: Chest x ray: lateral view with the Dynamic stent in place | Figure 17: Dedicated Dynamic stent introducer |
Given all the advantages and potential disadvantages of these two groups of stents, the optimum choice is determined by the anatomy of the lesion and the airway. The preferences and experience of the surgeon also plays a major role. There are some settings in which the morphology and position of the lesion (tortuous long strictures or lesions in proximity to lobar orifices) may be impossible to treat with silicone stents, and expandable stents provide the only remedy. Patients with airway malacia may require an expandable stent due to the difficulty of seating a silicone stent in the absence of a fixed stenosis.
We generally place a 14 to 16 mm (external diameter) silicone stent in the trachea and a 10 to 12 mm stent in the main bronchi. The experience of the surgeon greatly helps in choosing the correct size. The outer surface of the stent should adhere to the airway without pushing too much on the mucosa to avoid granulation formation. The correct length of the stent is selected by putting the tip of the rigid bronchoscope at the end of the stenosis and withdrawing it, measuring the distance at the level of the teeth. The stent should cover all the stenosis and 0.5 cm of normal airway at each end.
Stent Insertion
At the University of Rome “La Sapienza” we prefer silicone Dumon stents (Figure 9) for the vast majority of the lesions. We have placed expandable metal stents only when satisfactory placement of a silicone prosthesis could not be obtained or marked external compression produces distortion. (Figure 18) Silicone stents may be difficult to deploy. We routinely use the Dumon stent delivery system (EFER, La Ciotat, France). These stents are placed inside a delivery tube of the appropriate caliber based on the size of the rigid bronchoscope used for dilation and a plunger system is used to push it out of the introducer. Fine adjustments after stent deployment are performed with grasping forceps (Figure 19) under direct visualization.
There are also other techniques for silicone stent placement. The stent can be placed outside the rigid bronchoscope with an endotracheal tube inserted as a sheath over the proximal portion of the bronchoscope. The patient is then intubated with the bronchoscope–stent–pusher apparatus (Figure 20). The endotracheal tube prevents the stent from sliding upward during insertion of the bronchoscope. After placing the tip of the bronchoscope beyond the stenosis the rigid tube is gradually withdrawn while the external endotracheal tube is held in place, leaving the stent at the desired location.
A silicone stent can also be delivered through the lumen of the rigid bronchoscope, pushing it through the tube itself with the grasping forceps. With this technique the stent is usually deployed beyond the stenosis and needs to be pulled upward with forceps. Some larger stents (more that 16 mm or carinal Y stents) are placed through the vocal cords into the proximal airway using grasping forceps, and subsequently pushed in the correct position under direct control through the bronchoscope.
Expandable metallic stents have different delivery systems: the Wallstent and Ultraflex stent are contained within a delivery sheath; the Palmaz and Strecker stents need expansion over a balloon. Even if fluoroscopy guidance is recommended, simultaneous viewing through the rigid telescope or the flexible bronchoscope may contribute to improved accuracy of deployment. In some cases it is necessary to place multiple stents to optimize palliation.
Management of Stent
There are no studies supporting any medical intervention after stent placement and each center has his own policy. We usually recommend immediate saline nebulization, adding steroids for the first three to four days. Antibiotic prophylaxis is administered for three days. It is extremely important to keep the patient well hydrated to reduce the thickness of secretions; this may not be easy in cachectic patients with advanced cancer. A chest x-ray is always performed after stent placement and fiberoptic bronchoscopy is usually performed within two to three weeks unless the patient shows symptoms requiring earlier endoscopic monitoring. Afterwards, bronchoscopy is performed according to the underlying disease, the clinical status of the patient, and the presence of symptoms (respiratory failure, cough, stridor, purulent secretions, etc).
Preference Card
- Dumon–Harrell rigid bronchoscope with barrels of different diameter
- Adult length rigid telescope
- Long pediatric diameter telescope (better when working with bronchoscopes of small diameter)
- Dumon delivery system for silicone stent placement
- Dumon suction catheters
- Balloon dilators
- Rigid grasping forceps
- Rigid biopsy forceps
- Any flexible fiberoptic bronchoscope with a large working channel
- Biopsy forceps for the flexible bronchoscope
- Laser for tumor ablation (we use a Nd:YAG laser)
- Dumon stents of different diameter and length (tracheal and bronchial). Keep at least one Y stent for the carina
- Expandable metallic stents (either Wallstent or Ultraflex)
Tips & Pitfalls
- Before starting any endoscopic procedure, even if only an “evaluation” fiberoptic bronchoscopy is planned, have all the required tools ready available: rigid bronchoscope, laser, stents; inform the operating room personnel and the anesthetist.
- Work with a team of anesthetists and nurses familiar with operative endoscopy. It is better if they are always the same. The endoscopist / surgeon is responsible for solving all the potential problems, but he/she works much better if the entire team knows what to do.
- Keep in mind that there are oncologic patients with airway narrowing who are near the end of life. Sometimes there is no benefit in performing a difficult endoscopic procedure to give a patent airway to a patient who has exhausted all the available therapeutic resources and has no more strength to breath.
- Always keep different sizes and types of stents available, including at least one Y carinal stent. Remember that the Montgomery T tube is still useful and keep it in your armamentarium. When you use a stent be certain that it is immediately reordered so that one is readily available for future use. Keep informed of what you have or don’t have in your stent inventory.
- Do not rely upon an endoscopic examination performed by others unless there is a photograph available. If the endoscopy was not preformed recently, repeat it before planning treatment.
- Do not rely upon virtual bronchoscopy alone to plan an endoscopic procedure or to exclude the chance of surgery.
- Radiological imaging should be recent. Get a chest x-ray not more than one or two days before treatment.
- Always remember that surgical options are still the best treatment when technically feasible, according to the clinical status of the patient and the appropriate oncologic indications.
Results
The results of airway stenting strictly depend on appropriate patient selection, the location of the stricture, and the underlying pathology. Overall, more than 90% of the patients show satisfactory or excellent results. In case of neoplastic invasion of the airway, the endoscopic procedure is almost always intended to palliate symptoms and improve quality of life for a reasonable time. The natural history of the disease is difficult to predict and stop, and eventually airway obstruction will recur, often in association with other fatal complications. The more distal the neoplastic invasion is located within the airway, the more difficult is to obtain adequate and stable control.
Complications are still present and are related to the type of stent placed. There are no studies comparing the use of expandable and silicone stents in the airway. Five percent of the Dumon stents become obstructed by secretions and 1% to 2% by a granuloma formation at one of the edges of the prosthesis. Five percent to 10% of patients experience migration of the stent requiring repositioning. Overall, 10% to 40% of patients require more than one endoscopy to manage complications. Colonization of the inner stent surface is not infrequent and requires adequate antibiotic therapy (systemic and aerosolized) and often stent replacement.
Expandable metal stents (Wallstent and Ultraflex stents) are more often used and allow the same rate of improvement (>90% of the patients) as silicone stents. Migration is rare. The formation of granulation tissue is more frequent (15% to 30%) and it is usually located at the level of the uncovered edges (when covered stents are used). Tumor ingrowth may lead to stent occlusion when uncovered stents are used. A small percentage of patients may suffer from complications related to the inappropriate length of the stent; also fatigue fracture has been described. Special problems arise in removal of obstructing expandable wire stents. The stent may be plucked out wire by wire bronchoscopically; an extended transsternal linear tracheotomy may be required to remove uncovered stents, but the posterior portion of the stent incorporated into the membranous wall should be left in place to avoid a tracheoesophageal fistula. In these situations a permanent long T tube could be useful. Grillo repeatedly stressed the importance of considering all therapeutic options, especially for patients with benign disease. The lengthening of tracheobronchial strictures and the production of additional complications by using expandable stents have been recently reported [11,12]. Silicone stents may also produce additional injury, but this is often reversible after removal of the stent.
In conclusion, when facing a lesion causing central airway obstruction we must always consider all the potential therapeutic options. The potential benefits and risks of airway endoscopic treatment and stenting should be carefully considered by the endoscopist/surgeon. Decisions should be taken on a case–by–case basis considering the underlying disease and its natural history, the anatomy of the airway, and the clinical status of the patient.
References
- Cooper JD, Todd TRJ, Ilves R, Pearson FG. Use of the silicone tracheal T – tube for the management of complex tracheal injuries. J Thorac Cardiovasc Surg 1981;82:559-68.
- Cavaliere S, Venuta F, Foccoli P, Toninelli C, La Face B. Endoscopic treatment of malignant airway obstructions in 2008 patients. Chest 1996;110:1536–1542.
- Stephens KE, Wood DE. Bronchoscopic management of the central airway obstruction. J Thorac Cardiovasc Surg 2000;119:289–296.
- Venuta F, Rendina EA, De Giacomo T, et al. Endoscopic treatment of lung cancer invading the airway before induction chemotherapy and surgical resection. Eur J Cardiothorac Surg 2001;20:464–467.
- Venuta F, Rendina EA, De Giacomo T, et al. Nd:YAG laser resection of lung cancer invading the airway as a bridge to surgery and palliative treatment. Ann Thorac Surg 2002;74:995–998.
- Venuta F, De Giacomo T, Rendina EA, Trentino P, Della Rocca G, Ricci C. Double stents for carcinoma of the esophagus invading the airway. Ann Thorac Surg 1997;63:1515–1516.
- Dumon JF, Cavaliere S, Jimenez JP, et al. Seven-year experience with the Dumon prosthesis. J Bronchol 1996;3:6–10.
- Wain JC. Postintubation tracheal stenosis. Chest Surg Clin N Am 2003;13:231–246.
- Wood DE. Bronchoscopic preparation for airway resection. Chest Surg Clin N Am 2001;11:735–748.
- Dumon JF. A dedicated tracheobronchial stent. Chest 1990;97:328–332.
- Gaissert HA, Grillo HC, Wright CD, Donhaue DM, Wain JC, Mathisen DJ. Complications of benign tracheobronchial stenosis by self–expanding metal stents. J Thorac Cardiovasc Surg 2003;126:744–747.
- Grillo HC. Stents and sense. Ann Thorac Surg 2000;70:1142.






Comments