ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Extended VATS Thymectomy for Myasthenia Gravis Extrapleural Pneumonectomy
Patient Selection
In patients with nonthymomatous autoimmune myasthenia gravis (MG) thymectomy is recommended as an option to increase the probability of symptomatic improvement or remission. There is a consensus that all adults with generalized symptoms should have thymectomy, particularly if they are younger than 55 years. In elderly patients, in children, as well as in pure ocular myasthenia, thymectomy has been shown to be beneficial at least in some series, although the surgical indications thus far remain controversial [1]. These general selection criteria are also applied to video-assisted thoracoscopic (VATS) thymectomy that can be performed through left or right approaches [2,3].
Relative contraindications for a left-sided VATS approach include patients with a history of pleurodesis in the left pleural cavity or those with severe cardiomegaly, in whom a right-sided approach is preferred.
Operative Steps
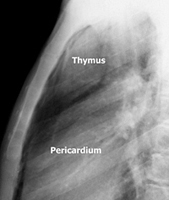
To facilitate the dissection and to shorten operative time, adjuvant pneumomediastinum is routinely performed 24 hours before the operation by introducing a Veress needle under local anesthesia at the level of the suprasternal notch, behind the posterior wall of the sternum [2]. Afterwards, 400 to 600 mL of air is insufflated in a sterile manner at a rate of 25 mL/min. Through this simple and safe maneuver, the thymus is progressively separated from the surrounding structures (Figure 1).
 |
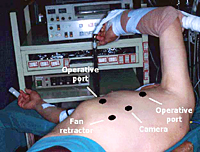 |
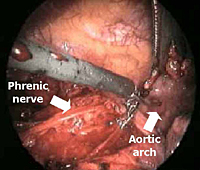
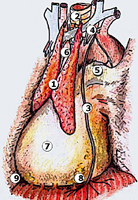
For the operation, after double-lumen intubation, the patient is placed in a 45-degree off-center position and 4 flexible thoracoscopic trocars are inserted (Figure 2). The entire hemithorax is carefully explored with particular attention to aortic arch, subclavian artery, pericardium, and phrenic nerve.
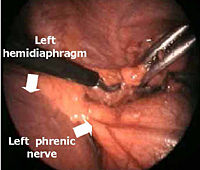
The dissection is begun inferiorly by incising the mediastinal pleura along the anterior border of the phrenic nerve (Figure 3). Because of the preoperative pneumomediastinum, the mediastinal adipose tissue is embedded with air and the thymus is already partially separated from the pericardium and from the sternum. As a result, the dissection proceeds more rapidly and easily, mainly by blunt maneuvers, with the aid of two pledgets.
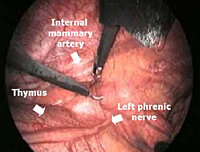
All mediastinal tissue including fat is swept away from the phrenic nerve and the left inferior horn is dissected off the underlying pericardium. Afterwards, the gland is dissected off the retrosternal area beginning just below the internal mammary pedicle and continuing rightwards until the right mediastinal pleura is visualized. The right inferior horn is fully dissected up to the isthmus (Figure 4).
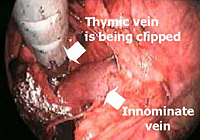
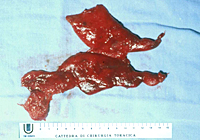
The lower half of the gland is then retracted upward and the thymic veins, usually two or three, are identified clipped and divided (Figure 5). Subsequently, the dissection proceeds cephalad, superior to the innominate vein, into the lower cervical region. The superior horns are progressively dissected free by blunt maneuvers with the aid of gentle traction applied downward on the thymus. In this way, even long and thin upper horns can be dissected en bloc with the rest of the gland. The intact thymus is extracted in a retrieval bag through the most anterior port and is examined to ensure that the whole gland has been removed (Figure 6).
 |
 |
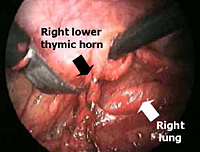
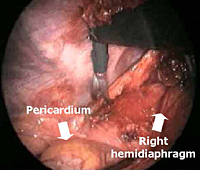
Subsequently, all mediastinal fat that is found in the pretracheal space, along the internal mammary pedicles, in the aorto-pulmonary window (Figure 7), as well as in the right (Figure 8) or left (Figure 9) pericardiophrenic angles that may contain ectopic thymic tissue is completely excised to accomplish an extended thymectomy (Figure 10).
At the end of the procedure one chest tube is inserted through the most medial port in the dissected mediastinal area.
Our current postoperative care includes immediate extubation, 24-hour ICU stay, removal of the chest tube after 24 hours, and hospital discharge between 36 - 48 hours provided that neurological examination is satisfactory.
Preference Card
- Flexible thoracoscopic Trocars: two 15mm, two 7 mm
- Scope - 0 degree rigid
- Endoscopic scissors
- Endoscopic dissector
- Endoscopic fan retractor
- Ring forceps
- Endoscopic cotton swab
- Medium endoscopic clip applier
- Specimen retrieval bag
Tips & Pitfalls
- VATS thymectomy should be performed following adequate training with radical transsternal thymectomy to gain familiarity with an extended removal of perithymic adipose tissue in the anterior mediastinum and lower cervical area that must be always pursued to maximize probability of remission.
- Select young and thin patients without large thymuses for the first cases.
- Avoid prolonged coagulation or excessive use of the fan retractor on the pericardium that can trigger dangerous ventricular arrhythmias requiring defibrillation. We have found an ultrasonically activated harmonic scalpel that minimizes the risks of electrically induced arrhythmias useful in the last several patients as an adjunct to sharp dissection and coagulation.
- Care should be taken to avoid vascular injuries that cannot be controlled endoscopically. For this purpose, when in proximity to major vessels such the innominate vein, we prefer delicate blunt dissection to sharp dissection or coagulation. On the other hand, minor vascular injuries can be easily controlled thoracoscopically.
- Nerve injuries can also occur during VATS thymectomy; in particular, care must be paid to avoiding phrenic nerve injury during dissection of perithymic adipose tissue in the left pericardiophrenic angle, and injury to the left recurrent nerve in the aorto-pulmonary window.
Results
In a recent series of 31 patients we had only one patient who required conversion to median sternotomy [4]. Mean operative time averaged 148 minutes, although in the last 10 patients it averaged 100 minutes. At pathologic examination ectopic thymic tissue was found in 10 patients (32%). It was located within the anterior mediastinal fat in 5 patients, in the aorto-pulmonary window in 3, and in the pretracheal or cardiophrenic fat in 2 patients each. Two patients had ectopic thymic tissue in two distinct locations. There was no operative death. One patient sustained a myasthenic crisis that required reintubation and mechanical ventilation for few days. Mean hospital stay was 5.2 days.
 |
In another recent prospective study, VATS thymectomy resulted in less pronounced impairment and faster recovery of pulmonary function than the sternotomy approach, leading the authors to conclude that VATS thymectomy could become their preferred surgical treatment of MG provided that satisfactory long-term results are confirmed [5].
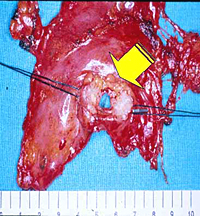
Patients with thymomatous MG are usually operated on through the transsternal approach; however, small (< 3cm in size) encapsulated thymomas incidentally discovered during VATS thymectomy can be safely excised en bloc with the thymic gland through this approach (Figure 11).
The cosmetic effect of VATS thymectomy is superior to that achievable by any other surgical approach. In our series, 26 (87%) patients judged cosmesis of their operation as excellent. Two patients had minimal thoracic pain for up to 6 months but none had thoracic pain at one year.
Long-term results of VATS thymectomy are still awaited, although intermediate results have been promising [4,6]. Remission and improvement rates of VATS thymectomy compared with the results achieved by other surgical approaches are reported in Table 1 [4,6-15].
Table 1
| Reference | Surgical Approach | No. of Patients | Follow-up duration (months) | Improvement Rate (%) | Remission Rate (%) |
|---|---|---|---|---|---|
| Bril[7] | TC | 52 | 101 | 90 | 44 |
| Calhoun[8] | TC | 100 | 60 | 85 | 35 |
| DeFilippi[9] | TC | 53 | 52 | 81 | 43 |
| Hatton[10] | TS | 52 | 46 | 58 | 28 |
| Masaoka[11] | TS | 375 | 60 | 88 | 43 |
| Stern[12] | TS | 56 | 82 | 81 | 53 |
| Jaretzki[13] | TS+TC | 95 | 40 | 93 | 38 |
| Ashour[14] | TS+TC | 40 | 14 | 87 | 48 |
| Bulkley[15] | TS+TC | 87 | 120 | 83 | - |
| Mack[16] | VATR | 33 | 23 | 88 | 19 |
| Mineo[17] | VATL | 31 | 39 | 96 | 36 |
TC=Transcervical; TS=Transsternal; TS+TC=Combined; VATR=right-sided video-assisted
thorascopic; VATL=left-sided video assisted thorascopic
 |
In our series, duration of disease of less than 12 months correlated with improved outcome at 24 months while preoperative Osserman class did not. This supports the hypothesis that the earlier the thymectomy, the better the outcome, independent of preoperative severity of symptoms.
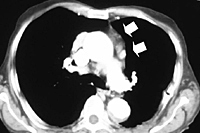
VATS thymectomy can also be employed to reoperate on patients with refractory MG who already underwent unsuccessful transcervical or sternal splitting thymectomy (Figure 12). This particular indication must still be considered investigational; however, it is worthy to note that in a series of 8 patients, we found at reoperation residual thymic tissue in all patients and 6 patients achieved sustained symptomatic improvement.
The optimal surgical approach to thymectomy remains controversial. Satisfactory results have been achieved by total and partial sternotomy, transcervical or combined transcervical-transsternal approaches, and video-assisted surgery.
Whatever the surgical approach, the accepted rationale is that beneficial effect of thymectomy is maximized by the removal of all thymic tissue, including ectopic thymic tissue, that can be scattered within the anterior mediastinum and cervical fat. Thus, it seems logical to assume that a less invasive but still radical approach is desirable and may facilitate the goal of early thymectomy that has been associated with improved outcome.
Our data indicate that VATS thymectomy is readily accepted by patients. The left-sided approach allows complete anatomic thymectomy and an extended resection of potential sites of ectopic thymic tissue. Morbidity with this approach is negligible, postoperative recovery is fast, and sustained symptomatic improvement can occur in more than 95% of the patients. Similar satisfactory results have been reported also by right-sided and more recently by infrasternal VATS approaches [17]. This suggests that the advantages of VATS thymectomy are valid in general, irrespective by the side from where the thymus is approached.
VATS thymectomy still needs confirmation by larger studies with long-term follow-up. Nonetheless, we are confident that it will have increasing use as a valid alternative to open approaches aimed at achieving a curative thymectomy in myasthenic patients.
References
- Gronseth GS, Barohn RJ. Practice parameter: Thymectomy for autoimmune myasthenia gravis (an evidence-based review). Neurology 2000;55:7-15.
- Mineo TC, Pompeo E, Ambrogi V, Sabato AF, Bernardi G, Casciani CU. Adjuvant pneumomediastinum in thoracoscopic thymectomy for myasthenia gravis. Ann Thorac Surg 1996;62:1210-2.
- Yim APC, Kay RLC, Ho JKS. Video-assisted thoracoscopic thymectomy for myasthenia gravis. Chest 1995;108:1440-3.
- Mineo TC, Pompeo E, Lerut T, et al. Thoracoscopic thymectomy in autoimmune myasthenia: results of the left-sided approach. Ann Thorac Surg 2000;69:1537-41.
- Rückert JC, Walter M, Müller JM. Pulmonary function after thoracoscopic thymectomy versus median sternotomy for myasthenia gravis. Ann Thorac Surg 2000;70:1656-61.
- Mack MJ, Landreneau RJ, Yim AP, Hazelrigg SR, Scruggs GR. Results of video-assisted thymectomy in patients with myasthenia gravis. J Thorac Cardiovasc Surg 1996;112:1352-60.
- Bril V, Kojic S, Ilse W, Cooper J. Long-term clinical outcome after transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1998;65:1520-2.
- Calhoun RF, Ritter JH, Guthrie TJ, et al. Results of transcervical thymectomy for myasthenia gravis in 100 consecutive patients. Ann Surg 1999;230:555-561.
- DeFilippi VJ, Richman DP, Ferguson MK. Transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1994;57:194-7.
- Hatton PD, Diehl JT, Daly BD, et al. Transsternal radical thymectomy for myasthenia gravis. Ann Thorac Surg 1989;47:838-40.
- Masaoka A, Yamakawa Y, Niwa H, et al. Extended thymectomy for myasthenia gravis patients: A 20-year review. Ann Thorac Surg 1996;62:853-9.
- Stern LE, Nussbaum MS, Quinlan JG, Fischer JE. Long-term evaluation of extended thymectomy with anterior mediastinal dissection for myasthenia gravis. Surgery 2001;130:774-9.
- Jaretzki III A, Penn AS, Younger DS, Wolff M, Olarte MR, Lovelace RE, Rowland LP. "Maximal" thymectomy for myasthenia gravis. Results. J Thorac Cardiovasc Surg 1988;95:747-57.
- Ashour M. Prevalence of ectopic thymic tissue in myasthenia gravis and its clinical significance. J Thorac Cardiovasc Surg 1995;109:632-5.
- Bulkley GB, Bass KN, Stephenson GR, et al. Extended cervicomediastinal thymectomy in the integrated management of myasthenia gravis. Ann Surg 1997;226:324-35.
- Pompeo E, Nofroni I, Iavicoli N, Mineo TC. Thoracoscopic completion thymectomy in refractory nonthymomatous myasthenia. Ann Thorac Surg 2000;70:918-23.
- Uchiyama A, Shimizu S, Murai H, et al. Infrasternal mediastinoscopic thymectomy in myasthenia gravis: surgical results in 23 patients. Ann Thorac Surg 2001;72:1902-5