ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Extrapleural Pneumonectomy
Patient Selection
Sarot first described the technique of extrapleural pneumonectomy (EPP) for the treatment of tuberculous empyema in 1949 [1]. During the last 30 years, EPP has been performed almost exclusively for malignant pleural mesothelioma (MPM). Early series reported high perioperative morbidity and mortality [2]. However, with improvements in patient selection, intraoperative management, and postoperative care, morbidity, hospital stay, and mortality have been reduced substantially.
The anatomical location, extent, and histology subtype of MPM determine if a patient should undergo an EPP. Several staging systems have been developed for MPM. The first and most widely used was developed by Butchart in 1976 [2]. In 1994, a TNM-based system was created by the International Mesothelioma Interest Group (IMIG) [3]. This system provides TNM descriptors and stage classifications. The most recent staging system (Brigham staging system) is based on analysis of over 180 patients treated for MPM at Dana-Farber Cancer Institute [4]. This system considers resectability, tumor histology, and nodal status (Table 1). Accurate preoperative staging is essential prior to an EPP. Only patients with early stage resectable disease should undergo EPP; patients with nodal disease (mediastinal or extrapleural) are not candidates for EPP.
Table 1. Revised Staging System for MPM
| Stage | Definition |
|---|---|
| I | Disease completely resected within the parietal pleural capsule (pleural, lung, pericardium, diaphragm, or chest wall containing previous biopsy sites) without lymph node involvement |
| II | All of stage I with positive resection margins and/or intrapleural (N1 or N2) lymph node involvement |
| III | Local extension into mediastinum, or chest wall, or through the diaphragm or peritoneum; or extrapleural or contralateral (N3) lymph node involvement |
| IV | Distant metastatic disease |
In MPM, tumor histology is the single most important factor influencing survival after tumor stage [4,5]. Epithelial cell type, the most common, confers the best prognosis; sarcomatous and mixed histologies are more aggressive, while desmoplastic MPM, the rarest subtype, is the deadliest. Most centers now operate only on patients with epithelial MPM because of the poor long-term survival after EPP for the other subtypes.
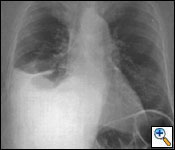
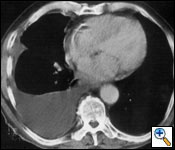
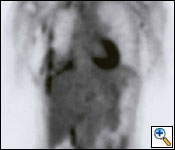
Radiological evaluation is essential to determine if a patient with MPM has potentially resectable disease. Posteroanterior and lateral chest roentgenogram (Figure 1), computerized tomography (CT scan) of the chest and upper abdomen (Figure 2), and magnetic resonance imaging (MRI) of chest have been the most widely used modalities. CT scan provides an estimate of tumor burden and extent of tumor both locally and distantly. MRI can supplement the CT scan for detection of tumor extension into the mediastinum or the abdomen. More recently, positron emission tomography (Figure 3) has been used to determine if a patient has early resectable disease based on no evidence of contralateral disease or distant metastasis. Early results are promising [6].
Other diagnostic modalities such as minimally invasive procedures may also be used to determine if a patient can undergo an EPP. Transesophageal echocardiography can be used to assess mediastinal involvement, especially of the heart or vena cava, and to perform fine needle aspirates of inferior mediastinal lymph nodes. Transthoracic needle aspiration via computerized tomography can also be performed to exclude extrapleural lymph node involvement (internal mammary lymph nodes). Mediastinoscopy is used frequently to determine nodal involvement if mediastinal lymph nodes (N2) are larger than 1.0 cm in diameter. Laparoscopic examination can also be used to identify transdiaphragmatic involvement that would preclude resection.
For a patient to be considered for an EPP, the patients should have a normal performance status. Pulmonary function tests, especially FEV1 and FVC, are used to determine if a patient can tolerate an EPP. For patients in whom a predicted postoperative FEV1 is less than 1 liter, a quantitative perfusion scan is useful to predict postoperative lung function after EPP. Arterial blood gases are obtained to rule out baseline hypoxia and/or hypercapnia. Two-dimensional dobutamine echocardiography is necessary to rule out ventricular dysfunction (EF < 40%), significant coronary artery disease, and pulmonary hypertension (mean pulmonary artery pressure > 30 mmHg) that may increase perioperative risks. An agitated saline study is also performed to determine if a patent foramen ovale is present that may result in a significant right to left shunt after EPP. Selection criteria are summarized in (Table 2).
Table 2. Selection Criteria
| 1. | Performance status 0 - 1 |
| 2. | Predicted postoperative FEV1 > 1.0 L |
| 3. | Room air PaO2 > 65 mmHg |
| 4. | Room air PaCO2 < 45 mmHg |
| 5. | Ejection fraction > 40% |
| 6. | Mean pulmonary artery pressure < 30 mmHg |
| 7. | Epithelial histology |
| 8. | No N2 or extrapleural lymph node involvement |
| 9. | No distant or contralateral disease |
| 10. | Ability to complete a trimodality treatment program |
Thoracoscopy has become the diagnostic procedure of choice with a yield of > 90% for MPM. Talc pleurodesis (aerosolized 5 gm) is usually performed at the time of the thoracoscopic pleural biopsy to prevent reaccumlation of a symptomatic pleural effusion. The talc pleurodesis facilitates extrapleural dissection at the time of EPP and may also prevent intraoperative spillage of malignant cells that may increase the risk of local recurrence. A single thoracoscopic access incision (Figure 4) is usually placed either in the 5th or the 8th intercostal space. The 8th intercostal access incision is preferred because it can be incorporated into a utility thoracotomy incision if necessary at the time of EPP to facilitate resection and reconstruction of the diaphragm. To take full advantage of the extrapleural edema created by the talc, I have found that it is best to proceed with the EPP within 2 to 3 weeks after pleurodesis. This also allows time to complete the staging work-up and to finalize the tissue diagnosis.
Operative Steps
Right EPP
After induction of general anesthesia, a left-sided double lumen endotracheal tube is placed and positioned fiberoptically. A nasogastric tube is placed to facilitate palpation of the esophagus during the posterior extrapleural dissection. Patients are monitored with an arterial line, continuous oximetry, central venous line, and urinary catheter. A thoracic epidural catheter is placed preoperatively and used for postoperative pain control. The patient is placed in the left lateral decubitus position.
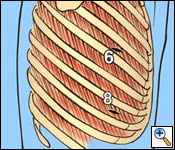
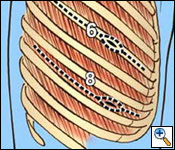
A standard posterolateral thoracotomy incision is carried out, making sure to incorporate all previous biopsy sites (Figure 5). The serratus muscle is saved and retracted medially. The chest is entered usually over the unresected sixth rib. The sixth rib may be removed if you prefer to do a single thoracotomy incision approach. I have found it to be beneficial to use a second lower thoracotomy incision on the right to facilitate resection and reconstruction of the diaphragm. After division of the intercostal muscles, an extrapleural plane is developed superior and inferior to the thoracotomy incision. Great care is taken during the extrapleural dissection not to enter the pleural cavity to prevent spillage of malignant cells within the operative field. The superior component of the dissection is carried out first (Figure 6). It is important not to injure the internal mammary artery and vein during the anterior dissection, which can lead to significant bleeding.
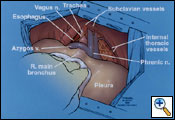 |
| Figure 6. The initial extrapleural dissection in the superior portion of the chest cavity. |
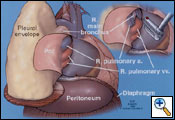
Combined sharp and blunt dissection continues toward the apex of the thorax. Medially the dissection is carried from the apex down to the azygos vein. The mediastinal pleural is then dissected free from the superior vena cava and azygous vein. The pericardium at the level of the azygous vein is opened to determine myocardial involvement; if no involvement is found, the pericardiotomy is extended anteriorly and inferiorly to encompass the tumor. Placement of your left hand inside the pericardium helps determine the extent of pericardial resection. Intrapericardially, the pulmonary artery is dissected free as well as the superior and inferior vena cava and the superior and inferior pulmonary veins (Figure 7). The dissection of the pericardium is completed at the pericardiopleural attachment overlying the junction of the inferior vena cava and hepatic veins.
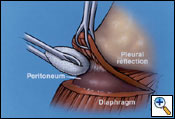
The diaphragm is excised starting at the anterior margin in a circumferential fashion working posteriorly (Figure 7). If possible, the peritoneum is not entered, preventing peritoneal seeding. Posteriorly, the peritoneum can be dissected free inferiorly with a sponge stick to separate it from the diaphragm (Figure 8). However, if the tumor burden is too great to avoid entrance into the peritoneum, the abdominal cavity is entered to help facilitate dissection at the level of the inferior vena and hepatic veins. If necessary, a lower utility thoracotomy incision is used at this point to help with removal of the diaphragm. This is usually placed at the site of the previous VATS access incision or chest tube site, which is excised to remove potential MPM implants. The diaphragm is divided over the inferior vena cava and the dissection is carried posteriorly leaving a rim of tissue if not involved by tumor to secure the reconstruction patch. After this maneuver, the diaphragmatic dissection is completed. The lung is retracted medially, and a complete mediastinal lymphadenectomy is carried out and the thoracic duct is ligated to prevent a postoperative chylothorax.
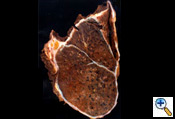
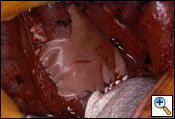
After completion of the en bloc dissection, 250 mg of SoluCortef is given intravenously. After approximately 10 minutes, the right pulmonary artery, superior and inferior pulmonary veins are ligated and divided with endoscopic 45 mm vascular staples intrapericardially (Figure 7). After the vessels are divided, the pericardium is opened posteriorly to the hilum, which completes the pericardial resection. The surgical specimen is then retracted anteriorly. The right mainstem bronchus is dissected free of peribronchial tissue and stapled with a 30 mm heavy wire stapler. The specimen (Figure 9) is removed and sent to the pathologist for inspection of resection margins, lymph node evaluation, and histological identification of the tumor. An intraoperative picture after removal of the en bloc specimen containing the lung, parietal pleura, pericardium and diaphragm is shown in (Figure 10).
After hemostasis is obtained, a tissue flap is developed to cover the bronchial stump. Options for tissue reinforcement include serratus muscle, intercostal muscle, thymic fat pad, or azygous vein. Pericardial fat is usually not available because of the extent of pericardium resected. Most commonly, I use a thymic fat pad (Figure 11), but if it is not available, I create an azygous vein bi-valved flap. The azygous vein is ligated and divided at the superior vena cava and only ligated, but not divided at its origin near the spine and bivalved to create an azygos vein flap to cover the bronchial stump (Figure 12).
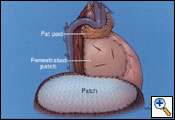
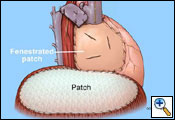
The pericardium on the right side is always reconstructed with a patch to prevent cardiac herniation. This is done with supple bovine pericardium (Bio-Vascular Inc., Saint Paul, MN) utilizing running and interrupted 2-0 polyprolene sutures (Figure 11). The key to prevent tamponade physiology is to use a large patch. The patch should also be fenestrated. It is important not to make the patch too narrow at the level of the superior and inferior vena cava because you may impair venous return.
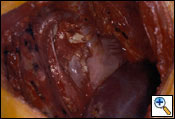
The diaphragmatic defect is closed with a 2-mm soft tissue polytetrafluorethylene patch (Gore-Tex, Inc., Flagstaff, AZ) using interrupted and running 1-0 polyprolene sutures to help prevent patch dehiscence (Figure 11). If there is not enough diaphragmatic tissue remaining, then the sutures are placed around the ribs inferiorly. Medially, the patch may be sutured to the pericardial patch to prevent impaired venous return of the inferior vena cava. It is extremely important to make sure that the reconstruction patch of the diaphragm is placed at or near the normal anatomical position to prevent intraabdominal contents from migrating upward into the chest cavity, thus putting them at risk for injury during postoperative thoracic radiotherapy. By utilizing the lower utility thoracotomy incision excellent exposure can be obtained for placement of the diaphragmatic patch. While placing the patch anteriorly a lighted retractor or disposable orthopedic light is used to ensure adequate bites of tissue are taken to help prevent dehiscence of the patch. An intraoperative picture after completion of the reconstruction is shown in Figure 13.
Areas of gross disease that cannot be resected following EPP are outlined with metallic clips for subsequent boost doses of radiotherapy. The chest cavity is sprayed with a thrombin solution to augment hemostasis. A 32 French right angle chest tube is placed and secured with 0-silk purse string. The chest cavity is then closed in the usual fashion to ensure an airtight closure. A chest x-ray is obtained in the recovery room to check for position of the heart and the mediastinal structures. The chest tube is removed and the pursestring is tied when the patient is breathing spontaneously.
Left EPP
The technique for left EPP is similar to that described for a right EPP. During the dissection of the posterior aspect of the specimen it is important to be in the correct plane in the periaortic region to avoid avulsion of intercostal vessels or injury to the aorta. It is also important to assess tumor involvement of the aorta at this time, because direct aortic involvement precludes resection. The left main pulmonary artery is dissected and divided extrapericardially if possible using endoscopic vascular staplers to prevent impingement of the main pulmonary artery. The pulmonary veins are dissected free and stapled intrapericardially. The left mainstem bronchus is dissected free to the carina to ensure a short bronchial stump and is stapled with a 30 mm heavy wire stapler. The left bronchial stump is usually not reinforced. Initially, I did not reconstruct the pericardium on the left side because the risk of herniation is low due to the native position of the heart within the left hemithorax. However, more recently I have been placing a bovine pericardial patch to allow near-normal position of the heart to minimize radiation exposure during adjuvant treatment, to help restore normal physiologic conditions, and to prevent development of a constrictive peel that may form with maturing of the left postpneumonectomy pleural cavity. The diaphragmatic defect is reconstructed with a 2 mm soft tissue polytetrafluorethylene patch. Great care is taken in reconstruction of the esophageal hiatus. Too large of an opening may result in intrathoracic herniation of the stomach and too tight of an opening may result in postoperative dysphagia. Therefore, the hiatus is reconstructed over a 50 Maloney dilator. If there is a paucity of tissue to anchor the patch at the hiatus, then smaller (3-0 or 4-0) polyprolene sutures are used to secure the patch to the periaortic tissue and or pericardium or pericardial patch. The chest cavity is closed in routine fashion, and the chest tube is removed when the patient is breathing spontaneously in the recovery room.
The postoperative management of these patients is similar to patients undergoing a standard pneumonectomy. However, because of reconstruction of the pericardium, especially on the right, the patient is at risk of developing hypotension upon arrival in the recovery room after being placed in the supine position. If this uncorrectable hypotension occurs, the patient may have too tight of a pericardial patch or a cardiac herniation secondary to a dehiscence of pericardial patch. The patient should be placed in lateral position and taken to the operating room immediately for resuturing of the patch if disrupted or enlargement of the patch if it is constrictive. The most important issues in the postoperative period are pain control and minimization of intravascular volume changes. Pain control is vital to minimize postoperative atelectasis and pulmonary dysfunction of the remaining lung. Thoracic epidural catheters are used for four days postoperatively. The patients are enrolled in a pulmonary rehabilitation program, which starts on postoperative day number two. A negative fluid balance is maintained postoperatively to prevent postpneumonectomy pulmonary edema. Aggressive pulmonary toilet is also carried out to prevent this possible fatal complication. Atrial arrhythmias are treated aggressively to prevent cardiac dysfunction. The average hospital stay is usually 5 to 10 days.
Preference Card
- Standard thoracotomy instruments (long)
- Lighted retractor
- Endoscopic 45 mm vascular staplers
- Bovine pericardial patch
- Polytetrafluorethylene 2 mm patch
- Long polypropylene sutures
- Spray thrombin
Tips & Pitfalls
- Minimize blood loss with careful ongoing meticulous hemostasis.
- Prevent pleural spillage by avoiding entry into the pleural space.
- Prevent peritoneal entry to minimize tumor contamination of the abdomen.
- Use interrupted and running sutures to minimize risk of patch dehiscence.
- Use a large pericardial patch to prevent tamponade.
- Place the diaphragmatic patch near normal position to prevent postoperative radiation injury to abdominal organs.
- Reinforce the bronchial stump (right) with viable tissue.
- Ligate the thoracic duct to prevent chylothorax.
- Use a utility thoracotomy incision inferiorly if necessary.
- Avoid too wide or too narrow hiatial reconstruction.
- Poor fixation of polytetrafluorethylene patch can lead to patch disruption and herniation of abdominal organs.
- Excessive bleeding before division of the pulmonary vessels increases the risk of postoperative complications such as postpneumonectomy pulmonary edema.
Results
EPP operative morbidity and mortality have decreased significantly from the earlier series because of improved patient selection and intraoperative care [7]. The first group to document this reduction was Sugarbaker and colleagues in 1999, who reported an operative mortality of 3.8% in 183 patients, which is comparable to the mortality of a standard pneumonectomy [4]. Even with this low operative mortality after EPP, the survival advantage for patients with MPM was not achieved until the introduction of trimodality therapy in 1991 [8]. Trimodality therapy usually consists of EPP followed by postoperative radiation and chemotherapy. By combining all three modalities, Sugarbaker and colleagues achieved a five-year survival of 15%; patients with epithelial MPM and negative lymph nodes and surgical margins had a remarkable five-year survival of 46% [4]. One of the drawbacks of trimodality therapy has been that a fair number of patients may not complete the adjuvant therapy because of prolonged recovery after EPP. To improve patient compliance, some centers are investigating using induction chemotherapy prior to EPP much like the clinical treatment protocols for locally advanced non-small cell lung cancer. Miller et al., showed that 90% of patients completed all three treatment modalities using induction chemotherapy followed by EPP and adjuvant radiotherapy [9]. Operative mortality was not increased, with only one operative death (3%) in this induction series; no survival data was reported.
Even though operative mortality has decreased significantly, postoperative morbidity is still a problem. Sugarbaker and others recently reported minor and major morbidity of 60% in 328 consecutive patients who underwent an EPP over a 13-year period; the most common complication was atrial arrhythmias [10]. Even in the induction series by Miller and colleagues the complication rate was 42%; again, atrial arrhythmias were the most common complication [9]. The best way to reduce complications and to minimize the overall impact on patient's outcome is by prevention with good patient selection, attention to operative detail, and heightened awareness and aggressive treatment of complications if they occur. The ideal treatment for MPM does not exist. Until it does, EPP as part of a trimodality treatment regiment provides the best method of significant tumor reduction that may prolong survival. This survival benefit can only be achieved with a low operative morbidity and mortality.
References
- Sarot IA. Extrapleural pneumonectomy and pleurectomy in pulmonary tuberculosis. Thorax 1949;4:173-179.
- Butchart EG, Ashcroft T, Barnsley WC, Holden MP. Pleuropneumectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24.
- Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma: From the International Mesothelioma Interest Group. Chest 1995;108:1122-1128.
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-65.
- Sugarbaker DJ, Mentzer SJ, Strauss G. Extrapleural pneumonectomy in the treatment of malignant pleural mesothelioma. Ann Thorac Surg 1992;54:941-946.
- Gerbaudo VH, Sugarbaker DJ, Britz-Cunningham S, Di Carli MF, Mauceri C, Treves ST. Assessment of malignant pleural mesothelioma with (18) F-FDG dual-head gamma-camera coincidence imaging: comparison with histopathology. J Nucl Med 2002;43:1144-49.
- Rusch VW. Treatment of malignant pleural mesothelioma. Semin Resp Crit Care Med 1997;18:363-373.
- Sugarbaker DJ and Garcia JP. Multimodality therapy for malignant pleural mesothelioma. Chest 1997;112:272S-275S.
- Miller DL, Vallieres EC, West HW, Okuno SH, Marks RS. Operative morbidity and mortality after induction chemotherapy and extrapleural pneumonectomy for malignant pleural mesothelioma. Presented at the 16th Annual Meeting of the European Association for Cardio-thoracic Surgery, Monte Carlo, Monaco, September 2002.
- Sugarbaker DJ, Richards W, Jaklitsch MT, et al. Prevention, early detection and management of complications following 328 consecutive extrapleural pneumonectomies (EPP). Presented at the 83rd Annual Meeting of the American Association for Thoracic Surgery, Boston, Massachusetts, May 2003.