ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
The History of Pulmonary Lobectomy: Two Phases of Innovation
Introduction
In the 21st century, when video-assisted pulmonary resections are done safely and effectively throughout the world, it is easy to forget how recently lung surgery was extremely risky and often fatal. Although the major technological advances which facilitated the minimally invasive lobectomy occurred in the last hundred years, the concept of lung resection has existed for centuries. The progression to the current methodology required advances in anesthesia, ventilation, the double lumen endotracheal tube, surgical technique, a closed chest tube drainage system, imaging technology, and understanding of pulmonary anatomy and physiology. The establishments of acceptable mortality rates following pulmonary resection in the early 1900s, and then the emergence of video-assisted thoracic surgery almost a century later, were two periods of rapid progress in the performance of pulmonary lobectomy.
Evolution of Indications and Operative Technique
The concept of thoracic drainage for infection was present in ancient civilizations, but pulmonary resection was thought to be impossible until the 1200s. The earliest lung resections relied on exteriorization of the lung tissue. The procedure was attempted only in cases of traumatic wounds which led to lung herniation, or suppurative wounds of the chest wall and pleura that exposed the underlying lung (1, 2). Rolandus published the first documented case of successful lung resection in 1499:
Called to a citizen of Bologna on the sixth day after his wound, I found a portion of the lung issued between two ribs… [I] made an incision through the skin, the breadth of my little finger-nail away from the wound, all round it. Then, with a cutting instrument, I removed all the portion of the lung level with my incision. The wound resulting from this resection was closed by the blood issuing from my incision, and was dressed frequently with the red powder and other adjuvants. By the grace of God it cicatrised, and recovery took place. (3)
The treatment of war wounds has been described in many additional reference documents from the middle ages, including Feldtbuch der Wunderarztnet (1517) and The Workes of that famous Chirurgion Ambrose Parey (1634) (Figure 1) (4,5). There are additional anecdotal reports of lung hernias from this time period. In his Opera Omnia (1646), Haldanus recounted a case of pulmonary resection for a traumatic lung hernia by Roscius (1). Nicholas Tulp described his successful treatment of a similar lung hernia in 1674. The patient was a man who suffered a stab wound below the left nipple during a drunken fight, and awakened the next day to find a three finger-breadth segment of his lung protruding. After walking for two days to Amsterdam for treatment, Tulp “ligatured it and cut it off with scissors: it weighed three ounces” and the patient recovered quickly, left with only a residual intermittent cough (3). Haldanus depicted another similar procedure in his Opera Omnia, a case that was also mentioned by Roscius in 1606 and Heister in 1724. Frederik Ruysch described a sailor with a low chest injury resulting in protrusion of the lung. It was treated with ligation and serial dressing changes until the necrotic lung sloughed off and the patient recovered (1). Heister commented on two of these cases in his 1724 Chirurgie, stating the important principles of preventing air entry into the pleural cavity, suturing the lung to the wound edges, and delaying amputation of the strangulated lobe until the lung was adherent to the chest wall (1, 2). Charles Bell reported another case of ligature of herniated lung in 1786 (1).
Fig. 1: The wound man was used as a diagram of frequent injuries to guide surgeons. Pare, 1634 (4)
In 1821, the American surgeon Milton Antony performed one of the first resections of chest wall with lung (Figure 2). The patient was a 17-year-old boy who had fallen from his horse two or three years prior to presentation, injured his chest, and developed a chronic lung abscess. The surgery was performed in the patient’s home without anesthesia, with the assistance of Antony's “aged, experienced, and accurate surgeon and anatomist” friend Dr. Pugsley (6). Upon exploration of the wound Antony was alarmed to find he was “able to penetrate the right cavity of the thorax, with my first and second fingers, in every direction, to the full depth of three and a half inches without any resistance more than the pulpy substance…” (6). His approach was described as “to take away with my fingers, all the parts within my reach which were removable without violence, or the danger of producing hemorrhage in the event of my reaching near any sound vessel. The result of this was the removal of all the disorganised parenchyma of the lungs within the reach of my fingers (judged to be between one and two pounds in weight” (6). Antony raised the idea of thoracoplasty to address the residual open cavity, saying “[i]t is the entire removal of the ribs, from the fifth to the diaphragm, by carefully dissecting them from the muscles and common integuments, leaving these to fall in the cavity, and be applied to its opposite wall for adhesion,” but quickly dismissed the idea that one could such a major surgery (6). The procedure was not reintroduced for at least five decades (1, 8). Unfortunately, after an initial recovery and return to normal activities, the patient died from measles four months after his surgery (6).
In 1837, South African surgeon Forde described a young soldier who suffered a spear wound and then removed the spear, creating an incarcerated right lower lung hernia of approximately five inches in length. He described the treatment as “I therefore determined on its removal, for which purpose I applied a ligature tightly round it…left the protruded lung undivided, until the process of adhesion should take place… On the third day, the desired adhesion being perfect, and the separation nearly effected by the ligature, the piece of lung was cut off with scissors” (9). The patient was able to run and throw his spear again within two weeks (9). Hale and Grinnell reported successful treatment of similar cases in the mid-1800s with equally successful results (10, 11). Padget described multiple lung hernias caused by traumatic war injuries in various wars during the 1800s, including seven patients during the American Civil War. One of these soldiers presented with a small, strangulated hernia after suffering a gunshot wound to the chest and was treated with ligation: “[t]he hernia was about one inch in diameter, having escaped from an aperture which was very much smaller. It was completely strangulated, being quite black, and insensible to the touch. We applied to the neck of the hernia a strong silk ligature” (3).
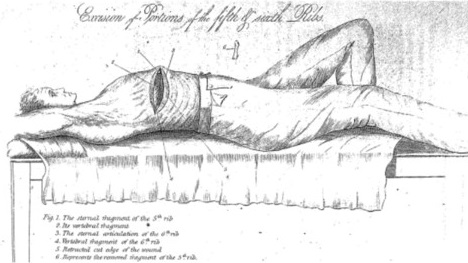 Fig. 2: Antony’s depiction of his patient who underwent partial resection of the fifth and sixth ribs, and a significant portion of the right lung. Antony, 1823 (7)
Fig. 2: Antony’s depiction of his patient who underwent partial resection of the fifth and sixth ribs, and a significant portion of the right lung. Antony, 1823 (7)
The first case of lung resection for a tumor and not a traumatic injury was reported in 1861. Pean successfully excised a lung tumor by suturing the pleura to the lung, then removing it with galvanic current cautery and dressing the wound with carbolic acid (12). Between 1881 and 1892, multiple additional attempts were made at lung resections in research animals and human subjects with variable results. In 1881, Glück reported a patient who underwent successful debridement and survived, similar to Antony’s case, and was encouraged about the potential applications by experimental animal surgeries (2, 13). Similar experiences were undertaken by Schmid, who ligated the pulmonary vessels and bronchi en masse initially, but then explored individual ligation of the hilar structures, a practice that would continue to be debated into the modern era (1, 12, 13).
In 1881, Block performed extensive experimentation in dogs, rabbits, pigs, and cows, and demonstrated that the pleural space was filled by the remaining lung tissue (13, 14). He, too, was hopeful about the potential to perform similar lung resections in humans, but was met with general pessimism when he presented his research at the German Surgical Congress in 1882 (14). Undeterred, Block proceeded. His female cousin was suffering from tuberculosis, and consented to surgery to resect both pulmonary apices. Unfortunately, she died during or immediately after surgery, and pathologic examination revealed normal lung. A legal investigation was initiated, and a despondent Block committed suicide (1, 12, 13).
Despite the promising advancements in animal studies, the death of Dr. Block’s patient led to a persistent skepticism about the feasibility of pulmonary resection in humans. An editorial comment at the time stated:
While these experiments may show that surgical interference with pulmonary disease is not so far removed from feasibility as generally supposed, and that pulmonary herniae may perhaps with safety be excised, we can hardly conceive of any one foolhardy enough to act on the suggestion of Dr. Block and attempt the excision of a tubercular pulmonary apex. (13)
In 1886, the Italian internist Forlanini recommended artificial pneumothorax for pulmonary collapse as the best treatment for tuberculosis on the rationale that because patient rest was essential for recovery from tuberculosis, perhaps rest of the diseased lungs wound be beneficial as well. He also acknowledged the technical difficulties of resection. Subsequently this collapse therapy became the preferred procedure for decades to follow (2, 15). However, animal research continued in the 1890s with emphasis on the necessity of individual ligation of the hilar structures, exploration of pathogenesis of adhesions between visceral and parietal pleura, the importance of antisepsis, and the use of different suture types (1).
Other attempts to treat human diseases with lung resection soon followed when French surgeon Tuffier in 1891 completed the first successful lung resection for tuberculosis. He employed chloroform anesthesia and a second intercostal space approach, and the patient recovered without complications. The patient was able to climb two flights of stairs on postoperative day eight to be presented at a surgical meeting (1, 12, 16). In 1893 in Great Britain, Lowson experimented with lung resections in rabbits and cadaveric dissections before operating on a patient with right upper lobe tuberculosis. He excised the anterior second and third ribs, externalized the diseased apex of the right lung, and resected a portion of lung “the size of half of a fist” (17). The patient survived and was discharged with a small bronchopleural fistula ten weeks postoperatively (18). In 1885, Doyen also successfully performed partial lung resections for tuberculosis, while Kronlein (1884) and Ruggi (1885) attempted similar procedures without success (1).
In 1895, MacEwen performed the first successful pneumonectomy, removing the entire left lung of a tuberculosis patient. He describes the removal of “160oz of pus, along with sloughs of the lung and caseated debris...By means of the finger and the long probe introduced into the cavity a dense layer of lung tissue with shreds of disintegrated lung were found still adherent to the chest wall.” After four weeks, the wound continued to drain, and the patient was brought back to surgery for thoracoplasty and eventually survived at least 11 more years with adequate quality of life and the ability to do light work (8). In the same decade, six patients with lung tumors (three sarcomas and three chondromas) underwent partial pneumonectomies with good results (1).
After the turn of the century, the development of antisepsis, anesthesia, and positive pressure endotracheal ventilation facilitated advancements in pulmonary surgery. Bronchiectasis, previously treated by serial incision and drainage, rib resection, induced pneumothoraces, and ligation of the pulmonary artery became a new indication for partial lung resection (1). In Germany in 1899, Gluck completed a left lower and partial upper lobectomy in a child with bronchiectasis with a good outcome (12). Heidenhain in 1901 performed a lobectomy for bronchiectasis in an adult, removing part of the left lower lobe of a 43-year-old man who had already undergone multiple incision and drainage procedures. Incidentally, a small carcinoma was found in the resected specimen. The patient developed a bronchopleural fistula, but otherwise recovered well (1, 2).
In 1908, Babcock resected the right lower lobe and segments of four ribs in a 20-year-old man. Although the patient died two weeks post surgery after apparently being neglected on a porch during a storm, Babcock comments that “the feasibility of delivering one more lobes of the lung through a posterior opening in the chest wall and the controlling of the blood vessels by ligatures applied directly to the hilum without recourse to the cautery was demonstrated” (19). Although these successful surgeries were encouraging, a lack of consensus on multiple variables, including type of anesthesia and ventilation, patient positioning, single versus multiple stage operation, and methods for control of secretions, slowed the progress in these early thoracic operations (1). The procedures were high risk and often complicated by infection and bronchial disruption (12).
In 1912, Davies operated on a 44-year-old man with a right lower lobe tumor, diagnosed radiographically, using a positive pressure anesthetic apparatus he had developed himself (Figure 3). He approached the tumor via the sixth intercostal space and performed individual hilar dissection and ligation, but unfortunately his patient died from an empyema on postoperative day eight. At autopsy, the bronchial closure was found to be intact (20). Single stage lung resection for bronchiectasis continued to have a high mortality rate, leading Mumford and Robinson in 1914 to question the one-stage approach, recommending “occasional successes do not prove that the one-stage amputation will become the method of choice. For the time being at least, the general surgeons should first produce compression of the lung by artificial pneumothorax or pulmonary arterial ligature; second, and much later, he should excise” (21).
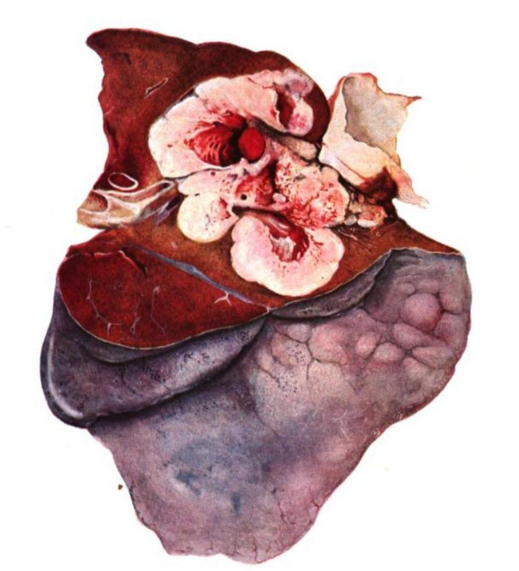 Fig. 3: Specimen from the resection of a right lower lobe primary bronchial carcinoma. Davies, 1913 (20)
Fig. 3: Specimen from the resection of a right lower lobe primary bronchial carcinoma. Davies, 1913 (20)
Despite a delay in progress of elective operations during World War I, in 1917, Robinson advocated for staged lobectomy. The initial stage involved rib resection to allow chest wall collapse, and then, one week later, the lung was mobilized, the hilum clamped, and individual ligation of the vessels and the bronchi performed to allow for necrosis and sloughing of the lung distal to the clamps over the subsequent seven to ten days. In the presence of excessive bleeding with mobilization, the completion of the procedure could be further delayed to a third stage. Bronchial closure proved to be difficult, and Robinson recommended use of a flap of skin and fat to close bronchial fistulas that developed (22).
The debate on the optimal staging of the procedure continued throughout the 1920s. In 1925, Graham described a multiple-staged pneumonectomy utilizing cautery. Even with complications including air embolism, delayed hemorrhage, empyema, and severe disfigurement, the procedure was thought by many to be safer than the one-stage lobectomy (23). He reported an overall mortality rate of 52% in 48 patients with bronchiectasis treated with pulmonary resection (23). Whittemore in 1927 agreed with the two-stage approach based on his experience: five of the six patients he treated with a one-stage approach died while four of five patients who underwent a two-stage procedure survived (24). His technique avoided the use of cautery by encircling the hilum with a catheter, exteriorizing the lobe, and suturing it to the wound margin which led to eventual sloughing and required no further management of bronchial pleura fistulae in the two patients who made complete recoveries. In 1930, Coryllos described a standardized multiple-stage lobectomy, consisting of artificial pneumothorax, phrenicotomy, thoracoplasty, and lobectomy via exteriorization or cauterization in relation to the successful cure of two patients with advanced bronchiectasis (25).
In comparison, Sauerbruch in 1920 reported a mortality rate of 100% in four patients who underwent one-stage lobectomy (1). Two years later, Lilienthal presented a series of 31 single-stage lobectomies with a 58% mortality rate, and commented on the importance of minimizing operative time, stating “any longer than forty-five minutes used in performing lobectomy means the almost certain loss of the patient” (18). Most of the deaths were attributable to empyemas, sepsis, pneumonitis, or hemorrhage (18). In 1929, Brunn described six patients who underwent one-stage lobectomy for bronchiectasis with only one death. He recommended hilar clamping, ligation, and suturing with phrenicotomy to keep the diaphragm immobile, creations of adhesions between the unaffected portion of lung and chest wall, as well as head down positioning, local anesthesia, and an intercostal incision (1, 26, 27). He was also the first surgeon to completely close the pleural cavity and chest wall and use an intercostal catheter for drainage (28).
In 1931, Ballon, Singer, and Graham presented a large series of 212 lobectomies with 72 deaths and 99 cures for bronchiectasis. They recommended a two-stage method involving removal of multiple ribs, ten-day interim period, and then at least one cauterization with a soldering iron followed by subsequent cauterizations, if necessary (1). Their technique was specifically intended for dissection of the left lower lobe hilum, but they correctly predicted that the anatomical dissection approach would be feasible in all lobes, except perhaps the left upper lobe, a qualification which proved to be unnecessary (2). The same year, Nissen performed the first complete pneumonectomy in a 12-year-old girl with history of a left main bronchus injury and subsequent mediastinal abscess, bronchial stricture, and extensive bronchiectasis development. The left lung was ligated with elastic cord and heavy silk sutures and sloughed off two weeks after the procedure. Her recovery was complicated by a bronchial fistula and small empyema that healed spontaneously (2, 27). Two years later, Haight reported a similar case with sloughing of the upper lobe by postoperative day sixteen, the lower lobe by postoperative day seventeen, and complete recovery of the patient (2).
In 1932, Janes and Shenstone presented 15 patients with bronchiectasis who underwent lobectomy using phrenic nerve crush or resection, regional or spinal anesthesia, and an incision through the sixth intercostal space, with a mortality rate of 26.7%. A modified tonsillar snare was used to surround the hilar structures; they noted that “nothing has given us such a feeling of comfort and safety in this operation as this simple device. Without devitalization of the tissue, it controls the blood supply, obstructs the bronchi, provides a solid support during the suture of the pedicle and in addition can be used in limiting mediastinal movement during the operation” (28). Two years later, Young of Glasgow performed another lobectomy with individual anatomic ligation; however in 1933 Roberts and Nelson reported ten lower lobe resections for bronchiectasis with mass ligation of the hilum, demonstrating the lack of consensus on hilar control (29).
In the 1930s it was also discovered that lung cancer was more amenable to surgical resection than bronchiectasis. In 1932, Churchill described four patients with carcinoma of the lung. Two of his patients had tumors that were unresectable at thoracotomy, and a third patient underwent lobectomy but died on postoperative day three. The fourth patient survived, and the resected specimen of two lobes revealed adenocarcinoma without lymph node infiltration. Churchill advocated for endotracheal anesthesia and individual hilar vessel dissection and ligation, but remained guarded with his predictions on successful lung resection for cancer treatment, stating “[i]t requires a courageous decision of optimism to suggest that the time will come when a complete resection of an early carcinoma of the lung with a careful dissection of the regional lymphatic nodes can be carried out with fair prospect of success” (30).
Graham performed the first complete pneumonectomy for lung cancer in 1933. The diagnosis had been made with radiography and pathology obtained from bronchoscopy. He hoped to perform only an upper lobectomy, but found an extensive tumor that required mobilization of the entire lung. He utilized an intercostal catheter to drain the pleural space, and the patient required subsequent drainage of an empyema and thoracoplasty with resection of the first and second ribs, but eventually went on to a complete recovery (31). Graham thought that removal of the entire lung was associated with a lower risk of cancer recurrence than lobectomy, stating “just as experience with carcinoma in other parts of the body has taught that the number of cures is, in general, directly proportional to the extent of radial removal, so it may be inferred, perhaps, that if the entire lung is removed the patient will have less chance of a recurrence than if only one lobe or a smaller portion is removed” (31).
Later in 1933, Rienhoff performed a pneumonectomy in a three-year-old girl with fibrosarcoma. He used anatomical dissection of the pulmonary vasculature and left main bronchus, but thoracoplasty and pleural drainage were not employed, and she recovered without complications (2, 27). In 1934, Freedlander successfully excised the right upper lobe of a patient with a tuberculous cavity who had failed collapse therapy (16). In 1935, Bohrer raised the idea of bilateral lobectomy for bronchiectasis during childhood in association with the report of a successful treatment of a seven-year-old boy (32).
In 1938 Crafoord employed positive pressure ventilation anesthesia for pneumonectomy (12). In 1939, Adams was ahead of his time as well, recommending endotracheal anesthesia, an intercostal incision, and posterior thoracic drainage. He controlled the hilum with a snare, amputated the distal lung, and sutured the vessels and bronchus separately (1). In 1939, Churchill and Belsey described a technique for resection of the lingua of the left upper lobe, an important emphasis on the segment and not the lobe as the surgical and pathological unit of the lung, stating that “a lobe is merely a segment of lung bounded by more or less constant and complete external fissures. It has been the convenience of these fissures rather than the underlying pathology that has defined the areas for pulmonary resection” (33). They suggested that “the bronchopulmonary segment may replace the lobe as the surgical unit of the lung” (33).
Individual ligation and suturing was initially recommended for total pneumonectomy, but was applied to lobectomy by Blades and Kent in 1940 after the dissection of 83 cadaveric lungs (34). Boyden’s meticulous study of bronchial and vascular anatomy contributed significantly to the refinement of lobectomy technique (35, 36, 37). Churchill and Klopstock followed in 1943 with a report of six successful upper lobe resections in patients with tuberculosis, a result that challenged collapse therapy. They commented that “[l]obectomy provides a more selective and intermediate method of eradicating certain lesions of tuberculosis than does collapse therapy… A method of treatment that combines conservation of lung function, with immediate conversion of the sputum, and a shortening of the span of treatment, cannot be dismissed until its scope has been more fully explored” (38).
World War II marked the identification of thoracic surgery as a separate specialty (1). During the 1940s and 1950s, most of the technical difficulties of lobectomy and pulmonary resection were overcome and the principle of lung tissue conservation was developed. Streptomycin was introduced in 1945, sparking the improvement of medical treatment of tuberculosis, and a subsequent decrease in surgery for tuberculosis (39). Further animal research and wartime surgical experience with traumatic bronchial transections facilitated the development of techniques for sleeve lobectomy (1). In 1947, Overholt and Langer furthered the idea of bronchopulmonary segment as the primary unit with “a new method… [that] does away with the use of clamps or suture of the lung surface. The development of the intersegmental plane is precise, relatively avascular, and not traumatic” (40).
The 1950s and 1960s brought continued emphasis on lung conservation and other important technical and medical advances. Chamberlain reported a larger series of 300 segmentectomies for tuberculosis with only nine deaths in 1953, and a few years later, in 1956, Thomas reported segmentectomy in three patients with carcinoma (41, 42). Nagel reported on the use of segmentectomy for tuberculosis in 1962 (43). Rasmussen, in 1964, and Bronfils-Roberts and Claggett, in 1972, published reports of segmentectomy for lung cancer with low mortality (1, 44). Sommerwerck, according to his 1970 account, frequently treated early lung cancers with segmentectomy (43). In the 1980s, there was debate over the adequacy of segmentectomy for curative treatment of lung cancer in terms of long term survival. Kutschera found a significant decrease in five year survival, while other reports found no survival difference in appropriately selected patients with small peripheral tumors who underwent segmentectomy (43, 45, 46).
The stapling device was first used for lung resections by Androsov and Potechina in 1955, and then Amosov and Berezovsky in 1961 (1). In 1962, Salzer demonstrated that lobectomy was equivalent to pneumonectomy for bronchial carcinoma, and lobectomy became the more commonly performed procedure (43). Advances in immunology led to the first lung transplantation in 1963 and refinements in technique continued in the 1970s and 1980s (47). Finally, after seven centuries of evolution, lobectomy could be performed safely with cancer as the most common indication.
A Delayed Entrance to the Modern Era of Minimally Invasive Lung Resection
The development of minimally invasive techniques for lung resection also followed a complicated and fascinating course. Although thoracoscopy therapy is commonly regarded as a modern technology, the groundwork was laid by Swedish physician Jacobaeus in the late 19th century at the same of the first elective open lobectomies were attempted (39). He developed thoracoscopy as a diagnostic technique to allow for inspection of the pleural cavity using a rigid cystoscope under local anesthesia with a candle as a light source (39, 48). In his landmark paper, he describes three important principles of the technique: minimal tissue trauma and pain with trocar placement, use of a transparent medium in the cavity, and the need for a scope thin enough to be placed through the trocar. In 1913, Jacobaeus described lysis of adhesions using the thoracoscope and cautery via a second incision, a procedure that was used for the next half century in the treatment of tuberculosis. The introduction of Streptomycin changed the treatment of tuberculosis, but in the 1950s thoracoscopy began to be used for lung biopsies (48).
It was not until four decades later that the combination of a light source and rod lens with micro cameras and video systems facilitated a high resolution panoramic view of the thorax (39, 48). The technique was originally used primarily for diagnostic procedures, but the mid-1980s success with therapeutic laparoscopic surgery led to application of the same principles to thoracic surgery (39, 48). The traditional posterolateral open thoracotomy is associated with significant, long-lasting pain, primarily from rib-spreading (39). Video-assisted thoracic surgery (VATS) provided the potential advantage of minimizing access and, therefore, postoperative pain; however safe dissection and control of the hilar structures was challenging, just as it was in the original lobectomy procedures one century before (39). In 1992, the first major meeting on VATS was held in conjunction with The Society of Thoracic Surgeons meeting (39).
The traditional open method of dissecting the pulmonary hilar vessels via the interlobar fissure, completing the fissures, and dividing the bronchus was applied to VATS lobectomy at first, but the fixation of the hilar vessels made their isolation and ligation dangerous. The original staplers did not articulate, adding another risk of vessel damage, and some surgeons attempted suture ligation of the vessels. In 1992, Lewis was one of the first surgeons to report a large series of 100 consecutive patients who underwent VATS lobectomies using four ports without rib spreading (39, 49). He stapled the hilar structures simultaneously without isolation. A large portion of the thoracic surgical community was skeptical of this simultaneous stapling technique, and individual isolation and ligation of the hilar structures continued, although there is no evidence of a difference in outcomes (39). With ongoing experience, it became clear that the anterior-to-posterior approach with control of the vein, artery, and then bronchus pioneered by McKenna was more effective (39, 50).
By the mid-1990s interest was growing, and it seemed that VATS was poised to take over as the preferred technique for many thoracic surgeries including lobectomy (39). This progress was interrupted by a series of reports, including a cross-sectional review from Landreneau in 1994 and a randomized prospective study from Kirby in 1995, which demonstrated no benefits in acute or long-term pain reduction from VATS compared to open thoracotomy (51, 52). These reports stalled the widespread acceptance of the VATS technique. In 1997, a survey of the General Thoracic Surgery Club demonstrated that 60% of respondents used VATS less than 20% of the time. Safety of hilar dissection in a closed chest, adequacy of oncologic outcomes, and cost were some of the concerns voiced at this time (53).
The wide variability in techniques and resultant skepticism about results created the need for strict standards to define VATS lobectomy. The Cancer and Leukemia Group B trial of 127 patients set the following criteria: avoidance of rib spreading, incision for specimen removal not to exceed 8cm, individual dissection of the vein, artery, and bronchus, and standardized lymph node sampling (54). This specific definition enabled the performance of research which proved the advantages of VATS over traditional thoracotomy. Compared to open thoracotomy, VATS has now been shown to reduce pain, decrease recovery time, decrease duration of chest tube use, decrease length of stay, minimize complications, and improve postoperative quality of life (39, 55). VATS is also associated with decreased effect on immune functions, potentially leading to improved long-term survival in lung cancer patients (39). Because of the low risk and excellent outcomes, it will be difficult for a new technology to supplant VATS as the standard of care for pulmonary lobectomy.
As experience with VATS lobectomy has grown, successful cases of chest wall resection (56, 57), bronchoplastic and sleeve resection (58), and pulmonary arterioplasty (59) have been reported. VATS segmentectomy for all anatomical segments, most commonly the posterior segments of the upper lobes and superior segments of the lower lobes, have also been described and are commonly performed by experienced thoracic surgeons (60). The minimally invasive approach has been associated with decreased blood loss, pulmonary complications, and length of stay, and comparable oncologic outcomes as the open approach (60).
There has been ongoing evolution of the minimally invasive technique in the 2000s. Use of the da Vinci robot system (Intuitive Surgical, Sunnyvale, CA) in minimally invasive thoracic surgery allows for 3D vision, improved dexterity, and steadier instrumentation, although it often requires placement of a fourth port (55). Robotic thoracic surgery has been slow to gain widespread acceptance due to the major limitations of cost, both for initial investment in the robot and ongoing purchasing of compatible instruments, and the absence of tactile feedback (39).
An advancement that is significantly less expensive and more widely accessible than robot-assisted surgery is needlescopic VATS. This modification uses video-thoracoscopes and instruments 2-3mm in diameter, and could further decrease pain associated with thoracic access (39). In another attempt to minimize trauma to the chest wall, Chinese surgeons have begun using only two ports for VATS lobectomy: one utility port for instruments and one camera port (55). This approach has also been popularized by D’Amico in the US (61). The next logical step would be uniportal VATS, an approach originally described by Rocco in 2004, which has been slow to gain widespread acceptance (62). The uniportal technique described by Sihoe of Hong Kong involves one 3-5cm incision in the anterior axillary line of the 5th intercostal space involving placement of the thoracoscope and all instruments through this port (55). The close proximity of the instruments to each other and the camera, and the lack of a posterior port requires an experienced surgeon and excellent cooperation from the assistant. These refinements have the potential for even more benefit to patients in shortened recovery time and decreased postoperative pain.
Although tuberculosis and trauma were the original indications for lung resection, they have been far surpassed by lung cancer in modern indications. Complications of tuberculosis, including failed medical management of multi-drug resistant tuberculosis and local cavitary disease or bronchiectasis, still warrant operative intervention in some patients, but are increasingly rare (63). New therapies including radiofrequency or microwave ablation, stereotactic radiosurgery, and stereotactic body radiation therapy have emerged as less traumatic and potentially curative approaches to lung cancer (39). As the field continues to evolve, thoracic surgeons will need to be comfortable performing open and minimally invasive pulmonary lobectomies and pay ongoing attention to emerging technologies.
References
- Kittle CF. The history of lobectomy and segmentectomy including sleeve resection. Chest Surg Clin N Am 2000;10:105-130.
- Lindskog GE. A history of pulmonary resection. Yale J Biol Med 1957;30:187-200.
- Paget S. The surgery of the chest. Bristol, England: John Wright & Co.; 1896.
- Pare A. The works of that famous chirurgion Ambrose Pare. 1st ed. London: Th. Cotes and R. Young, 1634. Accessed via Wikipedia Commons [Internet]. Available from: https://commons.wikimedia.org/wiki/File:Wounds_Man.jpg.
- McCallum JE. Military medicine: From ancient times to the 21st century. Santa Barbara, CA: ABC-CLIO; 2008.
- Antony M. Case of extensive caries of the fifth and sixth ribs, and disorganization of the greater part of the right lobe of the lungs; with a description of the operation for the same, &c. London Med Phys J. 1824;51:114-121. Reprinted from: Phil J Med Phys Sciences 1823;6:108-115.
- Antony M. Case of extensive caries of the fifth and sixth ribs and disorganization of the greater part of the right lobe of lungs with a description of the operation for the same, etc. Phil J Med Phys Sciences 1823;6:108-118. Accessed via reproduction in: Rutkow IM. The history of surgery in the United States, 1775-1900, Volume 2. 1st ed. Novato, CA: Jeremy Norman Co, 1992.
- MacEwan W. The Cavendish lecture on some points in the surgery of the lung. Br Med J 1906:2;1-7.
- Forde W. Case of removal of a portion of lung which protruded through a wound; etc. Med Chir Trans 1837;20:378-381.
- Housel W. Report of the medical society of Schuylkill County. Minutes of the Proceedings of the Medical Society of the State of Pennsylvania; 1855; Hollidaysburg, PA: Philadelphia: TK and PG Collins; 1855.
- Grinnell F. Removal of the lower portion of the left lung—recovery. Cincinnati Lancet Clin 1878;1:187.
- Chang JHT, Myers NA (Ed), Angerpointner TA (Ed); Historical Aspects, Paediatric Thoracic Surgery; New York, Springer-Verlag Berlin Heidelberg, 1991;25-29.
- Editorial: Resection of the Lung. Med News Philadelphia 1882;40:138.
- Walton GL. Letter from Berlin. Resection of the lung as proposed by Dr. Block. Boston Med Surg J 1883;108:261-262.
- Daniel TM. The history of tuberculosis. Respir Med 2006;100:1862-1870.
- Samson PC. Indications for lobectomy and pneumonectomy in pulmonary tuberculosis. Ann Surg 1940;112:201-211.
- Lowson D. A case of pneumonectomy. Br Med J 1893;1:1152-1154.
- Lilienthal H. Resection of the lung for suppurative infections with a report based on 31 operative cases in which resection was done or intended. Ann Surg 1922;75:257-320.
- Babcock WW. The operative treatment of pulmonary tuberculosis. JAMA 1908;50:1263-1265.
- Davies. Recent advances in the surgery of the lung and pleura. Br J Surg 1913;1:228-257. Figure available from: https://books.google.com/books?id=bFU9AQAAMAAJ&lpg=PA228&ots=OFUS8vPc7C&...
- Mumford JG, Robinson S. The surgical aspects of bronchiectasis. Ann Surg 1914;60:29-35.
- Robinson S. The resection of lobes of the lung. JAMA 1917;69:355-358.
- Graham EA. The surgical treatment of bronchiectasis. JAMA 1923;6:321-336.
- Whittemore W. The treatment of such cases of chronic suppurative bronchiectasis as are limited to one lobe of the lung. Ann Surg 1927;86:219-226.
- Coryllos PN. Treatment for bronchiectasis—multiple stage lobectomy. Arch Surg 1930;20:769-801.
- Smith A. Development of lung surgery in the United Kingdom. Thorax 1982;37:161-168.
- Nissen R. Development of total pneumonectomy. Am J Surg 1949;78:816-820.
- Shenstone NS, Janes RM. Experiences in pulmonary lobectomy. Can Med Assoc J 1932;27:138-145.
- Roberts JEH, Nelson HP. Pulmonary lobectomy. Br J Surg 1933;21:277-301.
- Churchill ED. The surgical treatment of carcinoma of the lung. J Thorac Surg 1932;2:254.
- Graham EA, Singer JJ. Successful removal of an entire lung for carcinoma of the bronchus. JAMA 1933;101:1371-1374.
- Bohrer JV. Lobectomy for bronchiectasis. Ann Surg 1935;102:1076-1078.
- Churchill ED, Belsey R. Segmental pneumonectomy in bronchiectasis. Ann Surg 1939;109:481-499.
- Faber LP. Individual ligation technique for lower lobe lobectomy. Ann Thorac Surg 1990;49:1016-1018.
- Boyden EA. The intrahilar and related segmental anatomy of the lung. Surgery 1945;18:706-731.
- Boyden EA. The anatomical hazards of lingulectomy. Surgery 1946;20:828.
- Boyden EA, Hartmann JF. An analysis of variations in the bronchopulmonary segments of the left upper lobes of fifty lungs. Am J Anat 1946;79:321-60.
- Churchill ED, Klopstock R. Lobectomy for pulmonary tuberculosis. Ann Surg 1943;117:641-669.
- Sihoe ADL, Cardoso (Ed); The evolution of VATS lobectomy, Topics in Thoracic Surgery;Shanghai, InTech, 2012;181-210. DOI: 10.5772/25429. Available from: http://www.intechopen.com/books/topics-in-thoracic-surgery/the-evolution...
- Overholt RH, Langer L. The technique of pulmonary resection. Springfield, MO: Charles C. Thomas;1951.
- Chamberlain JM. Segmental resection for pulmonary tuberculosis. Am J Surg 1955;80:673-681.
- Thomas CP. Conservative resection of the bronchial tree. J R Coll Surg Edinb 1956;1:169-186.
- Kutschera W. Segment resection for lung cancer. Thorac cardiovasc surgeon 1984;32:102-104.
- Bonfils-Roberts EA, Clagett OT. Contemporary indications for pulmonary segmental resections. J Thorac Cardiovasc Surg 1972;63:433-438.
- Hoffmann TH, Ransdell HT. Comparison of lobectomy and wedge resection for carcinoma of the lung. J Thorac Cardiovasc Surg 1980;79:211-217.
- Koga Y, Tomita M, Shibata K, et al. Evaluation of limited resection of the lung for treatment of lung cancer. Jpn J Surg 1982;12:1-5.
- Benfield JR, Wain JC. The history of lung transplantation. Chest Surg Clin N Am 2000;10:189-199.
- Loddenkemper R, Mathur PN, Lee P, et al. History and clinical use of thorascopy/pleuroscopy in respiratory medicine. Breathe 2011;8:145-155.
- Lewis RJ, Caccavale RJ, Sisler GE, et al. One hundred consecutive patients undergoing video-assisted thoracic operations. Ann Thorac Surg 1992;54:421-426.
- McKenna RJ. Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J Thorac Cardiovasc Surg 1994;107:879-882.
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy video-assisted thoracic surgery versus muscle-sparing thoracotomy: a randomized trial. J Thorac Cardiovasc Surg 1995;109:997-1002.
- Landreneau RJ, Mack MJ, Hazelrigg SR, et al. Prevalence of chronic pain after pulmonary resection by thoracotomy or video-assisted thoracic surgery. J Thorac Cardiovasc Surg 1994;107:1079-1086.
- Mack MJ, Scruggs GR, Kelly KM, et al. Video-assisted thoracic surgery: has technology found its place? Ann Thorac Surg 1997;64:211-215.
- Swanson SJ, Herndon JE 2nd, D’Amico TA, et al. Videoassisted thoracic surgery lobectomy: report of CALGB 39802—a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-4997.
- Sihoe ADL. The evolution of minimally invasive thoracic surgery: implications for the practice of uniportal thoracoscopic surgery. J Thorac Dis 2014;6:S604-S617.
- Hennon MW, Dexter EU, Huang M, et al. Does thoracoscopic surgery decrease the morbidity of combined lung and chest wall resection? Ann Thorac Surg 2015;99:1929-1935.
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-block chest wall resection. Eur J Cardiothorac Surg 2012;41:888-892.
- Li Y, Wang J. Video-assisted thoracoscopic surgery sleeve lobectomy with bronchoplasty. World J Surg 2013;37:1661-1665.
- Yu DP, Han Y, Zhao QY, et al. Pulmonary lobectomy combined with pulmonary arterioplasty by complete video-assisted thoracic surgery in patients with lung cancer. Asian Pac J Cancer Prev 2013;14:6061-6064.
- Ghaly G, Kamel M, Nasar A, et al. Video-assisted thorascoscopic surgery is a safe and effective alternative to thoracotomy for anatomical segmentectomy in patients with clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2016;101(2):465-72.
- Kara HV, Balderson SS, D’Amico TA. Modified uniportal video-assisted thoracoscopic lobectomy: Duke approach. Ann Thorac Surg 2014;98:2239-2241.
- Ng CSH, Gonzalez-Rivas D, D’Amico TA, et al. Uniportal VATS—a new era in lung cancer surgery. J Thorac Dis 2015;7:1489-1491.
- Madansein R, Parida S, Padayatchi N, et al. Surgical treatment of complications of pulmonary tuberculosis, including drug-resistant tuberculosis. Int J Infect Dis 2015;32:61-67.



