ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Ivor-Lewis Esophagectomy Without Left Gastric Pedicle Division
Index
INDEX
Ivor Lewis esophagectomy is occasionally required in patients who have previously undergone gastric surgery. A technique is described to salvage a gastric conduit by preserving the left gastric pedicle during esophagectomy.

Background
Gastric reconstruction of the esophagus following esophagectomy typically requires division of the left gastric pedicle to attain sufficient length for an esophagogastric anastomosis1. We report the case of an Ivor-Lewis esophagectomy with esophagogastric anastomosis while maintaining the left gastric pedicle.
Case Presentation
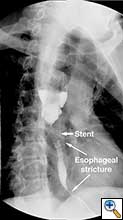
A 49 year old male presented to our institution with significant dysphagia and a 40 lb. weight loss secondary to a distal esophageal stricture. The patient had ingested lye 4 years previously and required partial gastrectomy, gastrojejunostomy, and partial colectomy at an outside institution at that time. His symptoms of dysphagia had progressively worsened despite repeated dilations and placement of an esophageal stent (Figure 1). Surgical reconstruction was therefore felt to be indicated. Preoperative computerized tomography angiogram did not clearly display the blood supply to the stomach, but did show that the colonic vasculature was adequate for use as a conduit if needed.
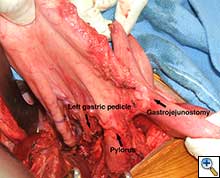
The patient underwent an initial midline laparotomy. After adhesiolysis, evaluation of the stomach revealed that both the right and left gastroepiploic arteries had been previously sacrificed. A wide Kocher maneuver was performed, which considerably mobilized the stomach and allowed for significant cephalad retraction. The left gastric artery, which was hypertrophied and appeared to be the primary blood supply to the stomach, was maintained. The stomach was then divided just distal to the gastroesophageal junction (Figure 2). A Heinecke-Mickulicz pyloroplasty was subsequently performed. It appeared that division of the gastrojejunostomy would not add to cephalad retraction of the gastric conduit. As such, the gastrojejunostomy was maintained.
The abdomen was temporarily closed, and the patient was placed in left lateral decubitus position. A right posterolateral thoracotomy was made in the sixth intercostal space. After mobilization of the esophagus, it was divided one centimeter distal to the azygous vein, and the stent in the proximal esophagus was removed. After the stent was removed an esophagoscopy revealed normal esophageal mucosa and no injury or stricture of the proximal esophagus.
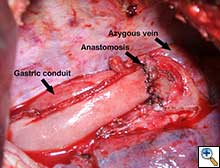
The gastric conduit was advanced into the thoracic cavity and rotated in an organo-axial fashion, such that the fundus was the most cephalad portion of the gastric conduit. With this rotation, the conduit had adequate length to reach the proximal esophagus and was not under tension. A hand-sewn, single-layered anastomosis was then performed between the esophagus and the gastric conduit (Figure 3).
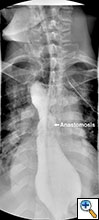
Figure 4. Postoperative barium swallow demonstrating free flow through the anastomosis with no residual stricture.
The thoracotomy was closed. The patient was placed back in supine position. The gastric conduit was secured to the diaphragmatic hiatus. The ultimate position of the pylorus was approximately 4 cm. inferior to the diaphragmatic hiatus. The abdominal fascia was closed.
The patient had an uncomplicated course. His nasogastric tube was removed on postoperative day five. A water-soluble esophageal swallow study on postoperative day six demonstrated normal flow of contrast through the anastomosis and stomach into the jejunum, with no evidence of leak or obstruction (Figure 4). The patient was discharged to home on regular diet on postoperative day eleven. Pathologic analysis of the specimen revealed multiple strictures within the esophagus, to a diameter as narrow as 5 mm, and no evidence of malignancy. The patient had complete resolution of his dysphagia on 9 month follow-up and was returning to his baseline weight.
Discussion
Ivor-Lewis esophagectomy with esophagogastric anastomosis has typically been described with division of the left gastric pedicle to provide sufficient length of the gastric conduit. Numerous patients who have undergone previous gastric surgery will present with need for esophagectomy, however2. Many of these previous operations will have resulted in division or interruption of the right gastroepiploic artery, the usual blood supply after esophagectomy.
In this clinical scenario, the stomach can still be used as the conduit, but the left gastric artery needs to be maintained to prevent gastric necrosis3. Our case shows that an esophagogastric anastomosis is still possible, but the stomach must be rotated in an organo-axial fashion more excessively than with standard esophagectomy. In our case, the anastomosis was ultimately performed to a more distal position on the gastric conduit than usual, within the fundus. This should not affect gastric emptying substantially but, given this extra rotation, a pyloroplasty should be performed.
Use of the stomach as a conduit is possible in patients after previous gastric surgery in which the left gastric artery is the main source of blood supply4. The ability to use the gastric conduit, in our case, was enhanced because our patient had a very “long” stomach. If the gastric conduit did not seem to have adequate length, however, then an alternative conduit, such as the colon or the jejunum, could have been used. Our patient had undergone a previous partial colectomy, but both preoperative and intra-operative evaluations suggested that the colon was a possible conduit, and would have been available if the stomach had not been of sufficient length. We feel that the length of the gastric conduit should be assessed prior to division of the left gastric pedicle in patients who have previously undergone gastric reconstruction and may have had interruption of their gastroepiploic arcade. If the length of the conduit is deemed inadequate, then either the left gastric pedicle can be divided or an alternative conduit can be used.
Clinical scenarios in which there is distal esophageal pathology5, either benign or malignant, are much more likely to allow for maintenance of the left gastric artery. In our patient the anastomosis was performed just one centimeter distal to the azygous vein without tension, but a cervical esophagogastric anastomosis probably would not have been possible. Preservation of the left gastric pedicle is expected to be difficult in patients who require very proximal anastomoses.
Many patients who have undergone previous gastric surgery will require esophagectomy for relief of a pathological condition. Previous operations may have divided the gastroepiploic arcade, but this does not absolutely rule out use of an esophagogastric anastomosis. Extra organo-axial rotation of the stomach is required, but this should not result in difficulty with gastric emptying, particularly if concomitant pyloroplasty is performed.
References
- Tomaszek S, Cassivi S. Esophagectomy for the treatment of esophageal cancer. Gastroenterol Clin 2009;38:169-81.
- Melstrom L, Bentrem D, Salvino M, Blum M, Talamonti M, Printen K. Adenocarcinoma of the gastroesophageal junction after bariatric surgery. Am J Surg 2008;196:135-8.
- Mittermair C, Klaus A, Scheidl S, Maglione M, Hermann M, Margreiter R, Nguyen N, Weiss H. Functional capillary density in ischemic conditioning: implications for esophageal resection with the gastric conduit. Am J Surg 2008;196:88-92.
- Palanivelu C, Prakash A, Senthilkumar R, Senthilnathan P, Parthasarathi R, Rajan P, Venkatachlam S. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position - experience of 130 patients. J Am Coll Surg 2006;203:7-16.
- Verschuur E, Kuipers E, Siersma P. Esophageal stents for malignant strictures close to the upper esophageal sphincter. Gastrointest Endosc 2007;66:1082-90.