ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Minimally Invasive Esophagectomy
Patient Selection
Conventional esophagectomy requires either a laparotomy with a transhiatal dissection or laparotomy combined with thoracotomy and is associated with significant morbidity and mortality. Reductions in complication rates have been reported in centers with a high volume of esophageal surgery but morbidity remains considerable. As advances in minimally invasive surgical instrumentation and technique continue, some surgeons have reported the application of minimally invasive techniques to resection of the esophagus in an attempt to further decrease the associated morbidity of esophagectomy. Most of these case studies or small series have used video-assisted thoracoscopy (VATS) to mobilize the thoracic esophagus in combination with a standard open laparotomy to complete the esophagectomy. Clear advantages of thoracoscopic esophageal mobilization over thoracotomy or laparotomy alone with transhiatal dissection were not demonstrated in these studies.
Patients in our institution are selected for minimally invasive esophagectomy (MIE) after CT scan, endoscopic ultrasound (EUS) and laparoscopic staging have excluded metastases and confirmed the presence of a resectable esophageal carcinoma. We previously performed a combined VATS/laparoscopic staging in patients without radiographic advanced disease; however, the VATS staging results have not influenced clinical management. Thus, for patients with potentially resectable esophageal cancer but with N1 or metastatic disease by EUS, we perform laparoscopic staging. We place an infusaport concurrently, if the patient has histologically node-positive disease and is a candidate for chemotherapy on a phase II trial.
Our early experience with MIE included primarily patients with Barrett's high-grade dysplasia or small tumors. This has evolved to include most patients with resectable lesions including those with limited nodal involvement. Neoadjuvant chemoradiation is not a contraindication for a minimally invasive approach. In some cases, patients with bulky tumors or extensive nodal involvement or those with prior surgery are approached via standard laparotomy and thoracotomy or with palliative measures.
Operative Steps
Esophageal Mobilization
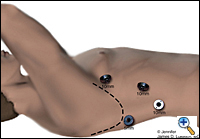 |
| Figure 1. Thorascopic port placement for MIE. |
The patient is intubated with a double lumen endotracheal tube (ETT) for single lung ventilation and positioned in the left lateral decubitus position. Four thoracoscopic ports are introduced (Figure 1). The camera port (10 mm) is placed at the seventh intercostal space, midaxillary line. A 10 mm port is placed at the eighth or ninth intercostal space 2 cm posterior to the posterior axillary line for the ultrasonic coagulating shears. Two additional ports are placed, one 5 mm posterior to the tip of the scapula and one 10 mm at the fourth intercostal space at the anterior axillary line for retraction and counter-traction during the esophageal dissection. Next, an 0 endostitch is placed in the central tendon of the diaphragm and brought out of the inferior, anterior chest wall through a 1-mm skin nick using the endo-close device. This traction suture allows downward retraction on the diaphragm without the need for a retractor and gives good exposure of the distal esophagus.
The mediastinal pleura overlying the esophagus is divided and the entire thoracic esophagus is exposed. First the inferior pulmonary ligament is divided to the level of the inferior pulmonary vein. The mediastinal pleura is divided anteriorly along the esophagus to the level of the azygos vein. While dissecting at the level of the subcarinal nodes, continuous attention is paid to avoid injuring the mainstem bronchi. The azygos vein is divided using the EndoGIA II vascular stapler. The vagus nerve is typically divided cephalad to the azygos vein. A penrose drain is placed around the mid-esophagus to facilitate traction and exposure. Circumferential mobilization of the esophagus with all surrounding lymph nodes and periesophageal tissue and fat is performed from the diaphragmatic reflection to the thoracic inlet. Large endoclips are used liberally on the lymphatics and aorto-esophageal vessels posteriorly to minimize bleeding and chylothorax complications. After complete mobilization of the esophagus, the penrose drain is placed through the thoracic inlet for later retrieval via the neck incision. An intercostal marcaine block is administered. A single 28 F chest tube is inserted through the camera port and the other port sites are closed with absorbable sutures.
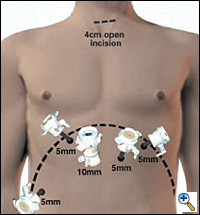 |
| Figure 2. Laporoscopic port placement for gastric mobilization in MIE. |
Gastric Mobilization
In the supine position, the double lumen ETT is exchanged to a single lumen tube, then 5 abdominal ports are placed on the anterior abdominal wall similar to the approach for a laparoscopic Nissen fundoplication: one cut-down 10 mm port in the right epigastrium and four 5 mm ports in the bilateral subcostal, left epigastrium and right flank locations (Figure 2). The left lobe of the liver is retracted upward to expose the esophageal hiatus using a Diamond- Flex retractor and held in place with a self-retaining system placed on left side of table. With the patient in maximal reverse Trendelenburg position, the dissection begins by dividing the gastrohepatic ligament and exposing the right crus of the diaphragm. The short gastric vessels are ligated using the ultrasonic shears and large endoclips. No clips are placed on the gastric side of the short gastric vessels to avoid potential displacement during the gastric pull-through. The dissection continues along the greater curvature of the stomach, preserving the right gastro-epiploic arcade. The stomach is retracted superiorly allowing lymph node dissection of the celiac and gastric vessels. Once the gastric artery and vein are exposed, they are divided using the EndoGIA II vascular stapler.
A pyloroplasty is then performed using ultrasonic shears and closed transversely using two traction 2-0 endostitches followed by approximately four interrupted 2-0 endostitches (Figures 3a-c).
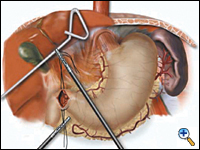 |
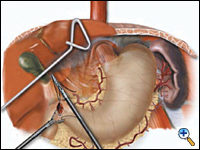 |
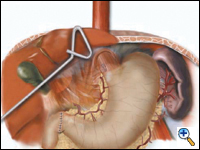 |
| Figure 3a. Creation of laparoscopic pyloroplasty. | Figure 3b. Closure of laparoscopic pyloroplasty | Figure 3c. Completed laparoscopic pyloroplasty |
The lesser curve fat and nodes are dissected en bloc with the stomach. The gastric tube is then constructed by dividing the stomach starting at the distal lesser curve, preserving the right gastric vessels and using the 4.8 mm endostapler (Figure 4). The gastric tube is attached to the esophageal and gastric specimen using two endostitches. An additional suture is placed on the anterior proximal gastric tube to prevent twisting as the tube is brought up to the neck.
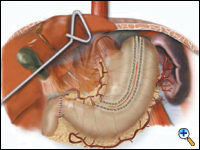 |
| Figure 4. Creation of 4 -5 cm gastric tube. |
A laparoscopic jejunostomy tube is placed by creating an additional 10 mm port on the mid right abdominal wall for the endostitch device, then attaching a limb of jejunum approximately 20-25 cm distal to the ligament of Treitz to the anterior abdominal wall using a 2-0 endostitch. The large needle from the Compat kit is placed percutaneously into the peritoneal cavity under direct laparoscopic vision and directed into the loop of jejunum. The guide wire and catheter are threaded into the jejunum. The jejunum is tacked to the anterior abdominal wall with a total of three to four 2-0 endostitches followed by one stitch several centimeters distally from the feeding tube to prevent torsion of the jejunal limb. The feeding tube is secured on the skin with several 2-0 silk sutures.
The last step in the abdominal operation is the final dissection of the right and left crural areas to open the plane into the thoracic cavity. This step is completed last to minimize loss of pneumoperitoneum into the mediastinum. We also divide the right and left crura to widen the hiatus to prevent gastric outlet obstruction.
Gastric Pull-up
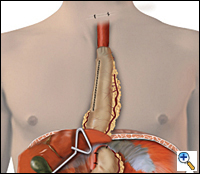 |
| Figure 5. Completed reconstruction after MIE. |
A 4 to 6 cm collar incision is made slightly to the left of midline above the suprasternal notch and the cervical esophagus is mobilized and exposed. Finger dissection is continued distally until the thoracic dissection plane is encountered. The esophagus is divided using the auto-pursestring device (typically size 45) and the esophagus is pulled up out of the wound. As traction is applied to the specimen from the neck, another surgeon laparoscopically guides the specimen in its proper alignment into the mediastinum. The specimen is removed from the field. An anastomosis is performed between the esophagus and the gastric tube using a 21- or 25-mm EEA stapler placed through a small anterior gastrotomy or handsewn. The gastric tube is reduced into the abdomen under laparoscopic guidance and approximated to the diaphragm anteriorly and bilaterally with three 2-0 endostitches. The completed reconstruction is shown in Figure 5.
Preference Card
Thoracoscopic esophageal mobilization:
- 30 degree 10 mm camera
- Endoretractor (10 mm) for retracting lung
- O Surgidac 48" endostitch and endoclose device for diaphragm retraction stitch
- Large endoclips
- Surgiwand suction
- ½" Penrose drain 18" cut in half
Laparoscopic mobilization:
- Padded footboard
- Bluntport 5-12 mm
- Versaport 5 mm (4)
- 30 degree 10 mm and 5 mm cameras
- Needle feeding jejunostomy kit (Compat Biosystems, Minneapolis, MN)
- Autosonix ultrasonic scalpel (USSC)
- Diamond-flex retractor and self-retaining system (Mediflex) retractor for left lobe of liver
2-0 Surgidac 7"endostitches cut to 3.5 - 4.5 " for pyloroplasty, feeding tube and gastric suturing
Tips & Pitfalls
- Place feeding jejunostomy routinely. If you don't have it, you will need it. We prefer the needle catheter feeding jejunostomy kit from Compat, although postoperative care of these small bore catheters requires avoiding medications that will clog them (essentially all medications) and careful suturing of the catheters to the skin to prevent dislodgement.
- Placement of a larger red rubber catheter feeding tube can be accomplished laparoscopically; however, the procedure is more time-consuming and results in a bulkier feeding tube.
- The recent appeal for creating a narrower gastric tube to improve gastric emptying has resulted in an increased rate of postoperative anastomotic leaks in our hands. We believe decreased anastomotic vascularity secondary to the narrower tube contributes to this complication. In addition a wider, more traditional gastric tube may provide more bulk at the thoracic inlet; thus, if a cervical esophagogastric anastomotic leak does occur, the enteral contents are less likely to contaminate the mediastinum.
- We performed a pyloromyotomy in 27 of our initial 77 patients but have found a pyloroplasty technically easier to perform laparoscopically.
Results
DePaula was the first surgeon to report a large series of 48 patients undergoing a total laparoscopic transhiatal esophagectomy. In two patients, conversion to open surgery was required and 2 others required VATS assistance. Swanstrom reported nine cases of laparoscopic total esophagectomy. There were no conversions to laparotomy but one patient required a right VATS with intra-thoracic anastomosis due to poor viability of the gastric tube. We have also reported this total laparoscopic approach but found that the completion of the thoracic esophageal mobilization and lymph node dissection was unsatisfactory and found the addition of VATS very helpful.
We have performed MIE in over 200 patients and have reported the results of our first 77 patients operated on from August 1996 to June 1999. Indications for MIE included esophageal carcinoma (n = 52), Barrett's esophagus with high-grade dysplasia (n = 19), and benign esophageal disorders (n = 6). The mean age was 65.1 years (range 30-89 years). Fifty patients (65%) had prior surgery involving the abdominal or thoracic cavity. Twenty of these patients had prior esophageal surgery: hiatal hernia repair in 4, laparoscopic staging for esophagus cancer in 16, laparoscopic Heller myotomy in 2. Conversion to laparotomy from MIE was necessary in 4 patients due to dense adhesions. There were no intraoperative emergencies requiring urgent conversion. Major complications occurred in 21 (27%) of patients. Six of the seven anastomotic leaks in the neck were managed by conservative measures. One developed a descending peri-gastric tube abscess that resolved with open neck drainage and drain placement. Minor complications in the peri-operative period occurred in 55% and included pleural effusions and atrial fibrillation.
Delayed complications (greater than 30-days post-operative) occurred in 4 patients. Two patients that had an initial pyloromyotomy developed persistent delayed gastric emptying requiring laparoscopic pyloroplasty with good results. One patient developed delayed gastric emptying with hold-up of contrast at the crural level. This required laparoscopic crural division with good results. One patient presenting with a moderately symptomatic diaphragmatic herniation of bowel into the right chest and underwent elective laparoscopic reduction and repair with good results.
Our initial results in 77 patients confirmed the feasibility and safety of performing esophagectomy using minimally invasive technique. Our technique continues to evolve and currently our preferred approach is the combined laparoscopic and VATS technique. The VATS approach improves our ability to perform a more extensive lymph node dissection and improves our ability to easily mobilize the middle and proximal third of the esophagus. Our average lymph node count is now approximately 20; this is in excess of what we routinely obtain with a transhiatal esophagectomy.
MIE is a technically demanding operation requiring advanced laparoscopic and thoracoscopic surgical skills. The routine performance of advanced laparoscopic and thoracoscopic procedures allowed us to perform this operation safely. There is a significant learning curve to this procedure as demonstrated by our initial operative times, which were frequently in excess of 7-8 hours. However, we have now demonstrated that surgeons in our group who have performed more than 20 of these procedures can accomplish the operation in under 5 hours. We recommend that any minimally invasive procedure on the esophagus only be performed by surgeons with extensive experience in open esophageal surgery and significant laparoscopic and thoracoscopic skills. Prospective trials with longer follow-up will be required to confirm any advantages of MIE over conventional approaches and open surgical approaches should remain the standard operation for esophagectomy in most institutions.
References
- Millikan KW, Silverstein J, Hart V, et al. A 15-year review of esophagectomy for carcinoma of the esophagus and cardia. Arch Surg 1995;130:617-624.
- Akaishi T, Kaneda I, Higuchi N, et al. Thoracoscopic en bloc total esophagectomy with vertical mediastinal lymphadenectomy. J Thorac Cardiovasc Surg 1996;112:1533-41.
- Dexter SPL, Martin IG, McMahon MJ. Radical thoracoscopic esophagectomy for cancer. Surg Endosc 1996;10:147-15.
- DePaula AL, Hashiba K, Ferreira EAB, et al. Trans-hiatal approach for esophagectomy. In: Toouli J, Gossot D, Hunter JG, eds. Endosurgery. New York, Churchill Livingstone, 1996, pp 293-299.
- Swanstrom LL, Hansen P. Laparoscopic total esophagectomy. Arch Surg 1997;132:943-949.
- Luketich JD, Schauer P, Landreneau R, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 1997;114:817-23.
- Luketich JD, Nguyen NT, Schauer P. Laparoscopic trans-hiatal esophagectomy for Barrett’s esophagus with high-grade dysplasia. JSLS 1998;2:75-77.
- Luketich JD, Nguyen NT, Weigel TL, et al. Minimally invasive approach to esophagectomy. JSLS 1998;2:243-247.
- Luketich JD, Schauer PR, Christie NA, Weigel TL, Raja S, Fernando HC, Keenan RJ, Nguyen NT. Minimally invasive esophagectomy. Ann Thorac Surg 2000;70:906-12.
- Nguyen NT, Schauer PR, Luketich JD. Combined laparoscopic and thoracoscopic approach to esophagectomy. J Am Coll Surg 1999;188:328-332.



