ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Postero-Lateral (Shaw-Paulson) Approach to Pancoast Tumor
Patient Selection
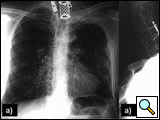
In 1932, the radiologist Henry Pancoast (Figure 1) first described a superior pulmonary sulcus tumor (SST) as a carcinoma (of uncertain origin) developing in the apex of the chest [1] (Figure 2). This tumor, located in the thoracic inlet, can invade the endothoracic fascia, infiltrating the lower roots of the brachial plexus, intercostal nerves, stellate ganglion, sympathetic chain, and adjacent ribs and vertebral bodies. The associated severe, unrelenting shoulder and arm pain along the distribution of the eighth cervical nerve trunk and first and second thoracic nerve trunks together with Horner’s syndrome (pupillary constriction, ptosis of the upper eyelid, and anhidrosis) has consequently been named "Pancoast’s syndrome." Currently, however, most reports of these tumors have included any patient with a tumor in the typical location of a Pancoast tumor, regardless of whether Horner’s syndrome or pain radiating down the arm is present [2-6]. Based on this evidence, Detterbeck [7] recently proposed a new definition of a Pancoast tumor: "A Pancoast tumor is a lung cancer arising in the apex of the lung that involves structures of the apical chest wall. Involvement of the chest wall only at the level of the second rib or lower should not be considered to meet the criteria for involvement of the apex of the chest. The chest wall involvement may be limited to invasion of the parietal pleura, or may extend deeper to involve the periosteum or the bone of the upper ribs or apical vertebral bodies, or it may include invasion of the subclavian vessels, the nerve roots of the brachial plexus, or the stellate ganglion." All other tumors in this location that are invasive above the level of the 2nd rib are termed superior sulcus tumor (SST). The Pancoast tumor accounts for less than 5% of all bronchogenic carcinomas, and recent reports indicate that its biology is similar to typical non-small cell lung tumors, with a predilection for distant metastases [8].
Before 1950, a nihilistic attitude predominated for this disease, widely considered inoperable and invariably fatal. Heburt and Watson [9] reported survival without treatment of between 3 and 24 months. In 1953, Chardack and MacCallum [10] reported long-term survival in a patient treated with lobectomy and chest wall resection followed by 65 Gy of irradiation. Subsequently, Shaw and Paulson [11] presented a series of 18 patients who were treated using preoperative radiotherapy (30 to 45 Gy on the primary tumor, supraclavicular region, and mediastinum) followed by en bloc resection. The good results led to consideration of radiotherapy-surgical approach as the treatment of choice for many years. Recently, preoperative concurrent chemotherapy and radiotherapy has been explored [6, 12-13]. Although no randomized trial has compared the two treatment modalities, the consistency of the data on preoperative chemoradiotherapy versus preoperative radiotherapy alone indicates that preoperative chemoradiotherapy represents a new standard of care for patients with Pancoast tumor.
Surgical Indications
The gold standard for the treatment of a Pancoast tumor is radical resection involving a lobectomy with removal of the invaded chest wall (including ribs, transverse processes, and all invaded structures, such as the lower trunk of the brachial plexus, stellate ganglion, and upper dorsal sympathetic chain). Absolute contraindications to surgery because the tumor is not suitable for radical resection include: the presence of extrathoracic metastases; invasion of the cervical trachea, esophagus, or brachial plexus above the C7 nerve root; extensive vertebral invasion; or invasion of the spinal canal through the intervertebral foramina. The presence of N2 disease, and involvement of the subclavian vessels, neural foramina, or a vertebral body, are relative contraindications to surgery. Although the resection of T4 Pancoast tumor has become possible owing to technical advances, the prognosis remains poor. In patients with involvement of the brachial plexus or spine, a combined thoracic, orthopedic, and neurosurgical approach can improve resectability and local control [14].
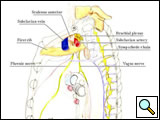
Several surgical approaches have been described for Pancoast tumor, ranging from posterior access [11] to anterior accesses [15-17], alone or in combination. The classic posterior approach popularized by Paulson and Shaw [11] gives excellent exposure of the posterior chest wall but does not allow safe, direct visualization and manipulation of more anterior anatomical structures forming the thoracic inlet. In particular, the management of invaded subclavian vessels (particularly the subclavian vein) is difficult and dangerous, often requiring an anterior approach. Therefore, a detailed knowledge of the anatomy of the apex of the chest and lower neck (Figure 3) and the availability of alternative approaches in their armamentarium are essential for surgeons managing these tumors.
 |
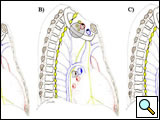 |
| Figure 3: Diagram of the thoracic inlet from a left lateral view. | Figure 4: The Pancoast tumor can invade the thoracic inlet in a posterior (A), middle (B) or anterior (C) site. |
Anatomical Remarks
A Pancoast tumor can invade the thoracic inlet in three different areas (Figure 4), producing clinical features with signs and symptoms related to the tumor location and requiring different technical considerations for the surgeon. Table 1 summarizes the compartments that constitute the thoracic inlet and the anatomical structures that the tumor can involve.
Table 1. Anatomical definition of the thoracic inlet and main clinical features in case of SST invasion.
| COMPARTMENT | LIMITS | ANATOMICAL STRUCTURES | SIGNS AND SYMPTOMS |
|---|---|---|---|
| Anterior | Between sternum and anterior edge of anterior scalene muscle. | Platysma, sternocleidomastoid andomohyoid muscles, jugular and subclavian veins, scalene fat pad. | Pain radiating the upper anterior chest wall, venous thrombosis. |
| Middle | Between anterior and middle scalene muscles | Anterior and middle scalene muscles, subclavian artery and primary branches, phrenic nerve, trunks of brachial plexus | Pain and parasthesia radiating to the shoulder and upper limb, arterial thrombosis, diaphragmatic paralysis |
| Posterior | Behind middle scalene muscle | Posterior scalene muscle, posterior scapular artery, posterior aspect of subclavian and vertebral artery, paravertebral sympathetic chain, stellate ganglion, nerve roots of brachial plexus, long thoracic and spinal accessory nerves, neural foramina, vertebral bodies and prevertebral muscles. | Pain in the axilla and in the medial part of the upper arm, Horner’s syndrome. |
Operative Steps
Incision
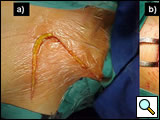
The patient is placed in the lateral decubitus position, with the arm over the head, exposing the axilla, scapular region, and neck. The incision starts anteriorly, allowing exploration of the chest cavity (usually through the fourth/fifth interspace) to assess the resectability. The extension of the tumor on the thoracic chest wall, thoracic inlet, lung, and mediastinum should be assessed. The incision is then extended posteriorly around the tip of the scapula and vertically upward between the spinous processes and posterior edge of the scapula, up to C7. The division of various muscle layers starts from the latissimus dorsi and trapezius to expose and subsequently divide the serratus anterior, rhomboidius major and minor, and levator scapulae muscles (Figure 5; Video 1). The rhomboid muscles insert into the medial border of the scapula, and care should be taken to avoid injuring the dorsal scapular nerve and satellite scapular artery, which run down the medial border of the scapula. A Fruchaud scapular retractor is used to elevate the scapula and expose the apex of the chest cage. The serratus posterior superior muscle is divided at its insertion on the second to fifth ribs, and the flap is preserved for later use (Figure 6; Video 2).
 |
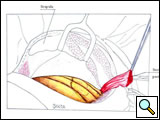 |
| Figure 5: Shaw-Paulson’s thoracotomy. | Figure 6: Diagram of the flap of serratus posterior superior muscle. |
Chest wall resection
The chest wall resection is carried out first, in order to release the involved chest wall into the pleural cavity allowing for a safer lobectomy. En bloc resection of the chest wall and lung is preferred to extrapleural dissection without rib sacrifice, which often leads to incomplete resection. A Tuffier rib spreader is placed between the undersurface of the scapula and the healthy underlying rib to better assess the limits of chest wall resection (Video 3). In most cases, the first two or three ribs are removed, although more ribs may be resected if required. The resection should guarantee large free margins, resecting 3-4 cm of uninvolved rib anteriorly and one rib and the intercostal muscle below the tumor inferiorly.
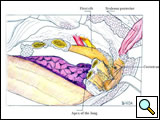
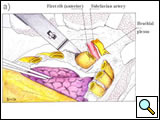
First, the anterior and inferior dissection is started along the established resection margins beginning with the healthy rib. The invaded ribs and intercostal muscles are divided using rib shears and electrocautery, in succession from below to above, and the intercostal neurovascular pedicles are ligated (Figure 7; Video 4). When the first rib is reached, the anterior and middle scalenus insertions to the second and first rib are divided with cautery, exposing the structures of the thoracic inlet crossing above the first rib. The scalenus posterior muscle is divided where it crosses the outer border of the first rib. The subclavian vein and artery and the brachial plexus are identified and protected by the operator’s finger (Video 5). Careful periosteal cleaning of the first rib anteriorly is performed, and the rib is divided in a tumor-free segment using a first rib shear or Gigli saw (Figure 8; Video 6).
 |
 |
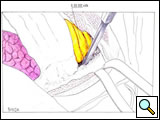 |
| Figure 7: Anterior resection of the 2nd and 3rd ribs. | Figure 8: Anterior resection of the 1st rib. | Figure 9: Diagram showing the disarticulation of the rib from the transverse process. |
The posterior phase of the dissection starts by incising the erector spinae muscle along its anterior border from T1 to T5 and retracting it outward to expose the costotransverse joint. If the tumor involves the parietal pleura only, with no rib or vertebral erosion, the ribs may be disarticulated from the transverse processes, preserving the latter structures. The head of the rib is disarticulated from the transverse process by dividing the costotransverse ligaments and, with gentle pressure, using a periosteal elevator to distract the rib anteriorly away from its transverse process attachments. (Figure 9; Video 7). The intercostal nerve and vessel originating from the intervertebral foramen are identified and divided between clips or sutured with 3-0 Prolene. This maneuver is repeated for each rib, until the first rib is reached. If the tumor involves the ribs posteriorly, the transverse processes are removed along with the adjacent lateral cortex of the vertebrae using an osteotome.
If uncontrolled bleeding occurs during dissection of the intervertebral foramen, the use of electrocautery with oxidized regenerated cellulose or absorbable gelatin sponges should be avoided or performed with caution to avoid dangerous consequences. A neural injury may result from cauterization in proximity to the spinal cord, and the migration of hemostatic drugs in the spinal canal can cause cord compression.
Dissection of the brachial plexus
The lower trunk of the brachial plexus is identified by retracting the first rib downward and can be dissected posteriorly until it splits into the C8 (above the neck of the first rib) and T1 (below the neck of the first rib) nerve roots. Most commonly, the neoplastic invasion is limited to the first thoracic nerve root, which may be divided medial to its entry into the lower trunk and lateral to tumor involvement, keeping the C8 nerve root intact. When the tumor involves the C8 nerve, the lower trunk of the brachial plexus should be divided medially, at its origin from the spine. The nerve roots are secured with clips or ligatures before transecting them at the intervertebral foramen to prevent cerebrospinal fluid leakage. If a leak occurs a muscle graft should be sewn over the leak using a small section of an intercostal muscle, erector spinae muscle, or serratus posterior superior muscle.
With a hand inside the chest, the first rib is cut either at its neck if the head is not involved with tumor or beyond the attachment of its tubercle to the transverse process. The chest wall is then released from the apex of the chest en bloc by sequentially dividing the lower portion of the stellate ganglion and first intercostal artery.
It is not usually necessary to reconstitute the posterior defect in the chest wall, as it is covered by the scapula. However, if more than three ribs are removed, leaving a large anterior or posterior defect, the defect can be reconstructed using Marlex mesh or a Gore-Tex patch to avoid an anterior flail chest or a trapped scapula.
Dissection of the subclavian vessels
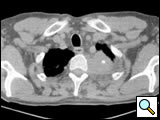
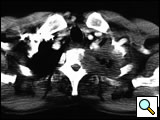
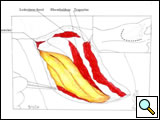
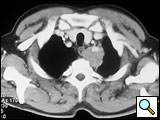
The subclavian artery can usually be dissected through the subadventitial plane (Video 8). Local branches (e.g., the internal mammary artery, thyrocervical trunk, or occasionally the vertebral artery) should be identified and transected if necessary. If the subclavian artery is invaded by tumor (Figure 10), the involved segment can also be resected and reconstructed through a posterior approach (Figure 11). After systemic heparinization, the artery is cross-clamped proximally and distally excluding the invaded segment and revascularized using either an end-to-end anastomosis or, more commonly, the interposition of a polytetrafluoroethylene (PTFE) graft, 6 to 8 mm in diameter (Video 9).
 |
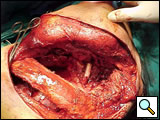 |
| Figure 10. Chest CT-scan shows the invasion of the subclavian artery by Pancoast tumor. | Figure 11. The invaded artery was resected and replaced by a PTFE graft through a posterior approach. |
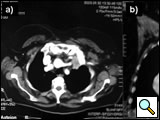
The anterior transcervical thoracic approach described by Dartevelle [15] or as modified by Grunenwald [16] should be added when the apical tumor involves the subclavian vein (Figure 12) that can be closed or replaced in the invaded segment and reconstructed using a reinforced PTFE graft (Figure 13). With anterior extension of the cancer from the apex downward to the innominate vein or superior vena cava (Figure 14), the anterior approach proposed by Masaoka [17] may offer a good exposure for surgery (Figures 15-16).
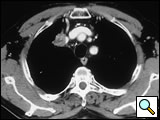
Vertebral body resection
Vertebral body invasion (Figure 17) has traditionally been considered a contraindication for surgical resection in SST. The combined efforts of thoracic surgeons and neurosurgeons specialized in spine stabilization, and recent advances in spinal instrumentation, have led to the development of surgical techniques for resecting vertebral bodies with malignant involvement. As a rule, up to one-quarter of the vertebral body may be removed without affecting the stability of the column. For SST extending into the intervertebral foramina without intraspinal extension, Dartevelle and others [18] described a technique for radical resection that combines the anterior transcervical and posterior midline approaches.
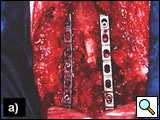
The classic posterior approach can be used to perform a total or partial vertebrectomy and neural foramina or transverse process resection [14, 19]. In patients with neural foramina or transverse process invasion only, the transverse process is drilled out with a high-speed diamond burr power drill, and the nerve roots are ligated and divided. If there is significant involvement of the surrounding osseous structures, a multilevel laminectomy and total or partial vertebrectomy are carried out through a posterior midline extension of the thoracotomy. The vertebrectomy defect may be reconstructed with autologous bone or a prosthesis. Anterior fixation is obtained with placement of an anterior locking plate and screw construct, and posterior fixation using rods fixed with screws and hooks is done with extensive resections and multilevel laminectomies (Figures 18-19). With a radical resection in the case of vertebral involvement, the transverse process and vertebral body must be resected first, before continuing the operation.
Pulmonary resection
A standard upper lobectomy with systematic lymphadenectomy is recommended for lung resection, even if the lesion is peripheral and small. This is usually performed through the hole previously made for the chest wall resection. As Ginsberg and colleagues [2] reported, lobectomy ensures better survival than limited resection (5-year survival 60 vs. 33%) and reduces the local recurrence rate.
Closure of the chest
Two apical chest tubes (32 or 28 Fr) are inserted; it is important to place the drainages not close to the apex of the chest since the chest wall in the resected part tends to collapse. Finally, the wound is closed, sequentially suturing the rhomboids, trapezius, serratus anterior, and latissimus dorsi muscles, and the overlying fascia with strong monofilament absorbable suture. The subcutaneous tissue and skin are closed with a transdermal suture. We prefer to keep the chest drainages under suction for at least three days postoperatively.
In postoperative management continuous and careful pain control should be obtained by using the epidural catheter placed before surgery and, if required, systemic analgesics to allow an early mobilization and an effective respiratory physiotherapy. Although the chest wall resection can be quite painful, usually pain control is good in these patients since the tumor invading the thoracic inlet has been resected.
Preference Card
- Headlight
- First rib cutter or Gigli saw
- Instrumentation for chest wall resection
- Fruchaud scapular retractor
- PTFE graft (6 to 8 mm diameter) for subclavian artery reconstruction
- Heavy titanium Castro-Viejo needle holder (for vascular sutures)
Tips & Pitfalls
- Obtain an accurate preoperative imaging study with an infused computed tomogram and magnetic resonance imaging to evaluate possible vascular and chest wall invasion.
- Ensure the surgeon has expertise in all approaches for Pancoast tumor.
- Provide wide tumor free margins in the chest wall resection (at least 2-3 cm anteriorly and one rib and intercostal muscle inferiorly) and obtain frozen section of the posterior resection margins.
- Perform careful superior dissection of the first rib to avoid nerve and vascular injuries.
- Ensure availability of an experienced neurosurgeon for vertebral resection and stabilization.
- A standard lobectomy with systematic lymphadenectomy is preferred to a lesser resection.
- Avoid the use of electrocautery and oxidized regenerated cellulose or absorbable gelatin sponges in the intervertebral foramina.
- Use posterior fixation of the vertebral column in case of a multilevel laminectomy or extensive vertebral body resection.
- Secure the nerve roots with clips or ligatures before transecting them at the intervertebral foramen to prevent cerebral spinal fluid leakage.
Results
The reported surgical mortality ranges from 2 to 8%, with morbidity rates between 7 and 38% [3, 10, 18, 23]. The most common surgical and postoperative complications include lung atelectasis caused by pain, chest wall resection, and sometimes phrenic nerve sacrifice; Horner’s syndrome and nerve deficits; hemothorax; spinal fluid leakage; chylothorax; and prolonged ventilatory support. The short- and long-term results after surgery combined with preoperative radiotherapy or chemoradiotherapy are summarized in Table 2.
The results of the prospective multi-institutional phase II trial (Intergroup trial 0160) [12, 20] of induction chemoradiation and surgical resection of SST and Pancoast tumor are impressive: 2% mortality, complete resection accomplished in 92% of operated patients compared with an average of 66% for historical series, a pathologic complete response in 33% of cases and residual minimal disease in an additional one-third of patients, and a local recurrence rate of only 23%. Therefore, many institutions consider preoperative chemoradiation to be the new standard of care for patients with SST.
Table 2. Principal experiences in surgical treatment of SST.
| Author | N° of patients | Preoperative treatment | Complete resection (%) | 2-year survival (%) | 5-year survival (%) | Mortality (%) |
|---|---|---|---|---|---|---|
| Paulson [24] | 62 | RT | 98 | NA | 34 | 3.3 |
| Devine [25] | 40 | RT | 70 | 20 | 10 | 8 |
| Sartori [3] | 42 | RT | NA | 38 | 25 | 2.3 |
| Maggi [26] | 60 | RT | 60 | NA | 17.4 | 5 |
| Ginsberg [2] | 124 | RT | 56 | NA | 26 | 4 |
| Martinod [6] | 139 | RT | 81.3 | 41 | 35 | 7.2 |
| Rusch [27] | 225 | RT | 64 (T3) | 59 | 46 | 4 |
| 39 (T4) | 33 | 13 | ||||
| Wright [28] | 20 | RT | 80 | 49 | 49 | - |
| 15 | CH+RT | 93 | 93 | 84 | - | |
| Martinez-Monge [13] | 18 | CH+RT | 76 | NA | 56 (4 yrs) | 16.6 |
| Kwong [29] | 36 | CH+RT | 97.3 | NA | 50 | 2.7 |
| Rusch [20] | 111 | CH+RT | 92* | NA | 44 | 2.4 |
Legend: NA= not available; RT= radiotherapy; CH= chemotherapy.
* A total of 83 patients were surgically treated.
The most important factors affecting the prognosis in SST patients are the completeness of resection and the absence of nodal involvement, Horner’s syndrome, and T4 stage [2, 21-23]. Some authors have reported encouraging results for patients with T4 apical tumors with invasion of the subclavian vessels [6, 15] or vertebral bodies [14, 18-19]. Owing to advances in surgical techniques, it is possible to achieve good long-term survival at experienced centers. Future refinements of the surgical technique may improve local resectability.
Acknowledgements: we thank Dr. Rino Binda for preparation of the diagrams and Dr. Abdel-Mohsen Hamad for the assistance in preparation of the manuscript.
Video 1
Video 2
Video 3
Video 4
Video 5
Video 6
Video 7
Video 8
Video 9
References
- Pancoast HK. Superior pulmonary sulcus tumour: tumour characterized by pain, Horner’s syndrome, destruction of bone and atrophy of hand muscles. JAMA 1932;99:1391–6.
- Ginsberg RJ, Martini N, Zaman, JG, et al. Influence of surgical resection and brachytherapy in the management of superior sulcus tumour. Ann Thorac Surg 1994;57:1440–5.
- Sartori F, Rea F, Calabrò F, Mazzucco C, Bortolotti L, Tomio L. Carcinoma of the superior pulmonary sulcus: results of irradiation and radical resection. J Thorac Cardiovasc Surg 1992;104:679–83.
- Wright CD, Moncure AC, Shepard JO, Wilkins EW Jr, Mathisen DJ, Grillo HC. Superior sulcus lung tumors: results of combined treatment (irradiation and radical resection). J Thorac Cardiovasc Surg 1987;94:69–74.
- Okubo K, Wada H, Fukuse T, et al. Treatment of Pancoast tumors: combined irradiation and radical resection. Thorac Cardiovasc Surg 1995;43:284–6.
- Martinod E, D'Audiffret A, Thomas P, et al. Management of superior sulcus tumors: experience with 139 cases treated by surgical resection. Ann Thorac Surg 2002;73:1534–40.
- Detterbeck FC. Changes in the treatment of Pancoast tumors. Ann Thorac Surg 2003;75:1990–7.
- Detterbeck FC. Pancoast (superior sulcus) tumors. Ann Thorac Surg 1997;63:1810–8.
- Herburt PA, Watson TS. Tumour of the thoracic inlet producing the Pancoast syndrome. Arch Pathol 1946;42:88–103.
- Chardack WM, MacCallum JD. Pancoast syndrome due to bronchogenic carcinoma: successful surgical removal and postoperative irradiation. J Thorac Surg 1953;25:402–12.
- Shaw RR, Paulson DL, Kee JL. Treatment of the superior sulcus tumour by irradiation followed by resection. Ann Surg 1961;154:29–40.
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for non-small cell lung carcinomas of the superior sulcus: initial results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Thorac Cardiovasc Surg 2001;121:472–83.
- Martinez-Monge R, Herreros J, Aristu JJ, Aramendia M, Azinovic I. Combined treatment in superior sulcus tumors. Am J Clin Oncol 1994;17:317–22.
- Grunenwald D, Mazel C, Girard P, et al. Radical en bloc resection for lung cancer invading the spine. J Thorac Cardiovasc Surg 2002;123:271–9.
- Dartevelle PG, Chapelier AR, Macchiarini P, et al. Anterior transcervical-thoracic approach for radical resection of lung tumors invading the thoracic inlet. J Thorac Cardiovasc Surg 1993;105:1025–34.
- Grunenwald D, Spaggiari L. Transmanubrial osteomuscular sparing approach for apical chest tumors. Ann Thorac Surg 1997;63:563-566.
- Masoaka A, Ito Y, Yasumitsu T. Anterior approach for tumors of the superior sulcus. J Thorac Cardiovasc Surg 1979;78:413–415.
- Fadel E, Missenard G, Chapelier A, Mussot S, Leroy-Ladurie F, Cerrina J, Dartevelle P. En bloc resection of non-small cell lung cancer invading the thoracic inlet and intervertebral foramina. J Thorac Cardiovasc Surg 2002;123:676-685.
- Gandhi S, Walsh GL, Komaki R, Gokaslan ZL, Nesbitt JC, Putnam JB Jr, Roth JA, Merriman KW, McCutcheon IE, Munden RF, Swisher SG. A multidisciplinary surgical approach to superior sulcus tumor with vertebral invasion. Ann Thorac Surg 1999;68:1778–85.
- Rusch VW, Giroux DJ, Kraut MJ, Crowley J, Hazuka M, Winton T, Johnson DH, Shulman L, Shepherd F, Deschamps C, Livingston RB, Gandara D. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 2007;25:313–8.
- Arcasoy SM, Jett JR. Superior pulmonary sulcus tumors and Pancoast's syndrome. N Engl J Med 1997;1370:337–343.
- Hagan MP, Choi NC, Mathisen DJ, Wain JC, Wright CD, Grillo HC. Superior sulcus tumors: impact of local control on survival. J Thorac Cardiovasc Surg 1999;117:1086–1094.
- Attar S, Krasna MJ, Sonett JR, Hankins JR, Slawson RG, Suter CM, McLaughlin JS. Superior sulcus (Pancoast) tumor: experience with 105 patients. Ann Thorac Surg 1998;66:193–198.
- Paulson DL. Carcinomas in the superior pulmonary sulcus. J Thorac Cardiovasc Surg 1975;70:1095–104.
- Devine JW, Mendenhall WM, Million RR, Carmichael MJ. Carcinoma of the superior pulmonary sulcus treated with surgery and/or radiation therapy. Cancer 1986;57:941–3.
- Maggi G, Casadio C, Pischedda F, Giobbe R, Cianci R, Ruffini E, Molinatti M, Mancuso M. Combined radiosurgical treatment of Pancoast tumor. Ann Thorac Surg 1994;57:198–202.
- Rusch VW, Parekh KR, Leon L, Venkatraman E, Bains MS, Downey RJ, Boland P, Bilsky M, Ginsberg RJ. Factors determining outcome after surgical resection of T3 and T4 lung cancers of the superior sulcus. J Thorac Cardiovasc Surg 2000;119:1147–53.
- Wright CD, Menard MT, Wain JC, Donahue DM, Grillo HC, Lynch TJ, Choi NC, Mathisen DJ. Induction chemoradiation compared with induction radiation for lung cancer involving the superior sulcus. Ann Thorac Surg 2002;73:1541–4.
- Kwong KF, Edelman MJ, Suntharalingam M, Cooper LB, Gamliel Z, Burrows W, Hausner P, Doyle LA, Krasna MJ. High-dose radiotherapy in trimodality treatment of Pancoast tumors results in high pathologic complete response rates and excellent long-term survival. J Thorac J Thorac Cardiovasc Surg 2005;129:1250-7