Introduction
There are several scattered reports in the English literature describing successful repair of aortoesophageal fistulae. In most cases, the patients have had prior surgery involving the esophagus [1] or the descending thoracic aorta [2], with an intrathoracic anastomosis in close proximity to the descending thoracic aorta. In some cases, however, aortic aneurysms have eroded into the esophagus [3] or esophageal foreign bodies have eroded through the wall of the esophagus into the descending thoracic aorta [4]. We present a case of aortoesophageal fistula caused by a ruptured aortic arch penetrating atherosclerotic ulcer, in the absence of previous esophageal resection, aortic aneurysm repair or esophageal foreign body.
Patient Presentation
A 67-year old woman presented to her primary care physician with a one-week history of solid food dysphagia. Her past medical history was significant for multiple myeloma with a recent relapse, for which she was undergoing outpatient chemotherapy at the time of presentation.
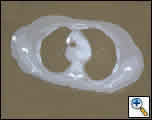 |
| Figure 1: CT scan of the chest at the time of presentation showing a pseudoaneurysm originating from the floor of the arch with compression of the periesophageal tissues. |
Upper endoscopy was attempted but quickly aborted since the esophagus appeared deviated toward the right side of the chest and bright red blood was suctioned from the oropharynx. A CT scan of the chest showed a pseudoaneurysm originating at the floor of the aortic arch [Figure 1]. She was urgently transferred to our institution for further care. She had two further episodes of hematemesis in the ambulance.
Upon admission to our intensive care unit the patient was obtunded and was immediately intubated. Her hematocrit was 21.6. Her blood pressure was 100/60 and her pulse was 120. She had a large bright red bloody bowel movement while a large bore intravenous line was being placed. She was emergently transferred to the operating room.
The chest was entered through a generous left thoracotomy at the fourth interspace. Multiple lytic lesions were found to involve the fourth and fifth rib, consistent with her diagnosis of multiple myeloma. She arrested just prior to opening of the pericardium and was urgently placed on cardiopulmonary bypass through the ascending aorta and the right atrial appendage. Upon institution of cardiopulmonary bypass, a normal sinus rhythm was regained and a large volume of O- blood and crystalloid were administered through the pump. Cooling was initiated and when the heart fibrillated a vent was placed through the left ventricular apex. No dissection was carried out during cooling in order to avoid inadvertent entry into the pseudoaneurysm.
With the patient’s bladder at 17oC the pump was shut off. The arch was fully dissected and opened and the fistulous tract was excised. The lumen of the distal ascending aorta and arch was heavily calcified. A 26mm Hemashield graft was beveled appropriately in order to replace the floor of the arch. The proximal anastomosis was then carried out using 3-0 polypropelene suture. Circulatory arrest time was 26 minutes.
The graft was clamped and after the appropriate maneuvers to eliminate air and particulate debris, perfusion of the brain and upper body was initiated. The distal anastomosis was then carried out in similar fashion. The graft clamp was removed and re-warming was initiated. She was separated from cardiopulmonary bypass without difficulty. Total cardiopulmonary bypass time was 117 minutes.
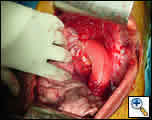 |
| Figure 2: Distal arch/descending thoracic aortic graft in place. |
The area of esophageal disruption from the pseudoaneurysm exceeded 3cm in length; thus no attempts were made to reconstruct the esophagus. The pericardium and an intercostal pedicle were used to separate the graft from the esophageal erosion [Figure 2].
Later that day, she was brought back to the operating room where she underwent esophagectomy, stapling of the esophageal remnant at the level of the diaphragm, cervical esophagostomy and gastrostomy tube placement through a separate laparotomy incision.
Her postoperative course was complicated by a cerebrovascular accident resulting in bilateral upper extremity and left lower extremity paresis. Her right lower extremity had normal motor function. Her sensation was intact throughout. MRI of the head revealed diffuse embolic strokes. She was extubated on POD #5 and maintained a normal mental status throughout her hospitalization. Her renal and hepatic functions remained normal, and she never displayed any signs of abdominal visceral ischemia. By the time of her discharge to a rehabilitation facility on POD #14 she had regained some motor function of her right upper extremity.
Comment
Aortoesophageal fistula is a devastating complication resulting from a variety of thoracic conditions, which is uniformly fatal unless promptly treated. A review of the literature reveals several primary causes for its development, with the instigating event arising from both the aorta [thoracic aortic aneurysm] or the esophagus (thoracic malignancy). The majority of presentations, however, are due to secondary causes (swallowing of foreign body, previous intrathoracic esophageal or aortic surgery). To our knowledge, this is the first report of an aortoesophageal fistula arising from rupture of an aortic atherosclerotic ulcer in the absence of aneurysmal disease. As expected, most reports in the literature are isolated case presentations, with the largest series being four patients in a single institution [3].
The principles of treatment remain the same, no matter what the cause is. Adequate resection of the involved aortic portion and re-establishing the integrity and continuity of the aorta should be the primary goal. Prevention of graft contamination is equally as important. This can be achieved either by direct repair of the esophagus and tissue interposition between the repair site and the aortic graft or by resection of the involved esophageal portion, proximal diversion and distal stapling with plans for future reconstruction. In our case, we had to resort to the latter option since the expanding pseudoaneurysm had devitalized a substantial length of the esophageal wall, thus rendering primary reconstruction impossible. A 6-week course of intravenous antibiotics is a necessary adjunct to surgical treatment. This can be empiric or guided by intraoperative cultures.
With the recent advances in intraluminal stent technology, a combination of aortic and esophageal stenting has been advocated as treatment of this problem [5]. Stent infection, migration and immediate availability have limited their use. In such patients as our own, endovascular repair would have not been an option despite the absence of aneurysmal dilatation, since the rupture point was in close proximity to the takeoff of the left carotid artery, and a bare metal stent would have left the fistulous track open. However, future evolution and immediate availability of branched stent graft technology may provide a consistent solution to patients suffering from this devastating complication.
References
- Molina-Navarro C, Hosking SW, Hayward SJ, and Flowerdew ADS. Gastroaortic fistula as an early complication of esophagectomy. Ann Thorac Surg 2001;72:1783–1788.
- Wickstrom PH, Streitz JM, Erickson RV, and Hoffman BDK. Repair of Aortoesophageal Fistula After Aortic Grafting. Ann Thorac Surg 1997;64:253-255.
- Michael J. Reardon MJ, Brewer RJ, LeMaire SA, Baldwin JC, and Safi HJ. Surgical management of primary aortoesophageal fistula secondary to thoracic aneurysm. Ann Thorac Surg 2000;69:967-970.
- Yamada T, Sato H, Seki M, Kitagawa S, Nakagawa M, and Shimazaki H. Successful Salvage of Aortoesophageal Fistula Caused by a Fish Bone. Ann Thorac Surg 1996;61:1843-1845.
- Léobon B, Roux D, Mugniot A, Rousseau H, Cérene A, Glock Y, and Fournial G. Endovascular treatment of thoracic aortic fistulas. Ann Thorac Surg 2002;74:247-249.



