ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Thoracoscopic Management of Spontaneous Pneumothorax
Komanapalli C, Sukumar M. Thoracoscopic Management of Spontaneous Pneumothorax. November 2022. doi:10.25373/ctsnet.21529062
Patient Selection
Patients who have had a second episode of spontaneous pneumothorax are those most likely to be considered for thoracoscopic pleurectomy and apical blebectomy. However, patients with a first episode of spontaneous pneumothorax who have a prolonged air leak (greater than 72 hours), incomplete expansion of the lung (Figure 1), bilateral pneumothoraces, associated hemothorax, tension pneumothorax (Figure 2), or a bleb on their CT scan (Figure 3) should be offered surgery at the time of the first occurrence [1]. Patients who have had a spontaneous pneumothorax and whose occupation places them at risk for a second episode or places them in a situation where medical intervention is not readily accessible should be considered for surgery. Therefore divers, pilots, submarine personnel, and those that work or live in the wilderness or space are suitable patients [1].
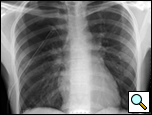 | 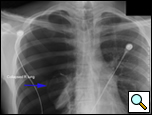 | 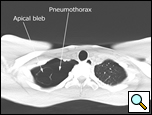 |
| Figure 1: Chest x-ray showing incomplete expansion of lung after chest tube placement. | Figure 2: Chest x-ray showing tension pneumothorax with mediastinal shift. | Figure 3: CT Scan showing apical bleb. |
Occasionally, extreme anxiety over placement of a chest tube or fear of another pneumothorax may be reason to perform surgery at the first episode of pneumothorax. A contralateral recurrence after an initial pneumothorax is an indication for bilateral surgery [2]. A relative indication for surgery is patients who have an asymptomatic apical bleb or suffered a pneumothorax on one side and are discovered to have a bleb on the contralateral side. If such patients are at risk due to their occupation or are in a situation where medical care would not be readily accessible, surgery may be considered.
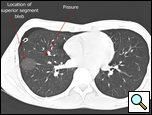 |
| Figure 4: CT scan showing location of superior segment bleb. |
Patients who have had a previous operation for spontaneous pneumothorax where complete pleurectomy or bleb resection was not performed and who develop a recurrence should be offered re-operation. Prior surgery for pneumothorax is not a contraindication to use of a thoracoscopic approach, as pleural adhesions are relatively few in these patients. A CT scan of the chest readily identifies blebs most commonly seen at the apex of the lung but also in the superior segment of the lower lobe (Figure 4, Video 1 below).
Operative Steps
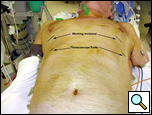 |
| Figure 5: Patient in supine position allowing access to both hemithoraces. |
The operation is performed under general anesthesia with single lung ventilation. The patient is placed in a straight lateral position as for a posterolateral thoracotomy. If both sides are being operated on, then each can be approached in this position sequentially, or the patient may be positioned supine so both sides of the chest are accessible without having to reposition the patient (Figure 5).
The thoracoscope is placed in the 7th ICS in the mid axillary line, anterior to the anterior superior iliac spine. A working incision is made in the 4th or 5th intercostal space between the mid axillary line and the anterior axillary line. The lung is collapsed and a ring clamp is used to grasp the apex of the lung and bring it into view (Video 2 below).
Once a bleb is identified it is held with a ring clamp and an EndoGIA stapler with a blue or green load (45/60mm length; 3.5/4.8mm staple) is introduced into the chest. It is passed under the ring clamp and is applied to the lung parenchyma inferior to the ring clamp to include a margin of normal lung parenchyma. The affected part of the lung is then resected by firing the stapler (Video 3 below). It is not necessary to use a buttress for the stapler as the patients are usually young with normal lung parenchyma. The superior segment of the lower lobe is inspected for any other blebs, as is the rest of the lung. If further blebs are identified, these are resected as described.
Next, a subtotal parietal pleurectomy is performed. This is begun by dividing the pleura in the line of the working incision (4th or 5th space) posteriorly, adjacent to the spine, and working anteriorly till the incision is reached. The posterior edge of the upper pleura is held in a ring or tonsil clamp, and a peanut dissector is used to develop an extrapleural plane (Video 4 below). Once this is well established, the pleura is stripped from the chest wall posteriorly, anteriorly, and laterally using a sponge stick (Videos 5, Video 6 below). The dissection proceeds to the apex of the chest where the pleura is then transected at its reflection with the mediastinal pleura (Video 7 below). Next, the inferior margin of the cut edge of the pleura is stripped and resected as described above (Video 8 below). The remaining mediastinal and diaphragmatic pleural surfaces can be abraded to irritate these surfaces using a Bovie electrocautery scratch pad held with a ring clamp (Video 9 below). This must be vigorous enough to remove some of the pleura in a piecemeal fashion and leave the rest of the pleural surface with multiple areas of punctate bleeders. Hemostasis must be meticulous and systematic after pleurectomy. The chest wall is visualized from the apex to the diaphragm using the sucker to ascertain bleeding points and any bleeding areas on the chest wall, diaphragm, and mediastinum are cauterized (Video 10 below). The chest cavity is then irrigated and two 28 Fr chest tubes are placed, one anteriorly and the other posteriorly, to drain the chest and help re-expand the lung (Video 11 below).
The chest tubes are maintained on suction for the day of the operation and then placed to water seal (Figure 6). Often, a small apical pneumothorax is seen which is usually of no significance and resolves over the next 2 to 4 weeks. Chest tube drainage can be high for the first 48 hours, after which a marked decrease is seen. If an air leak persists for more than 3 days, one of the tubes is left in the chest and the patient is discharged with a pneumostat device (Heimlich valve) or a minipleurevac if the chest tube drainage is more than 30cc/24 hours (Figure 7).
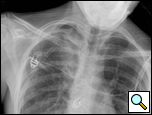 | 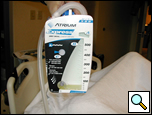 |
| Figure 6: Postoperative chest x-ray after blebectomy and pleurectomy. | Figure 7: Minipleurevac. |
Preference Card
- 30 degree thoracoscope
- Curved thoracoscopic ring clamp
- EndoGIA stapler
- Yankauer sucker
- Peanut dissector
- Extra Bovie scratch pad
- Tonsil Sponge stick
Tips & Pitfalls
- Carefully examine the CT scan for blebs preop
- Resect all blebs
- Perform a pleurectomy and vigorously abrade those areas that cannot be resected
- Drain the chest completely with 2 tubes
- Manage postoperative air leaks conservatively
Results
Thoracoscopic pleurectomy and blebectomy have become the standard of care in the management of spontaneous pneumothorax. The operation is 95-98% successful in preventing pneumothorax, with decreased morbidity as compared to posterolateral thoracotomy [3-6].
The indications for operation are now well established, and a more aggressive surgical approach is advocated since thoracoscopic intervention has a relatively low morbidity, a high rate of prevention of recurrent pneumothorax, and is permanent. A CT scan of the chest is indicated in patients with a pneumothorax to look for blebs; this directs the surgeon to the bleb(s), blebectomy being a vital part of the operation [7].
The technique of axillary thoracotomy, apical pleurectomy, and blebectomy is comparable to thoracoscopy in terms of efficacy and morbidity, but does not provide access to the whole chest [8, 9].
Bilateral operations can be performed under the same anesthetic without difficulty by approaching the pleural cavities through their respective sides [10, 11]. A technique of performing bilateral blebectomy through a unilateral approach has been described, but the ability to perform a pleurectomy is not known [12]. A uniportal approach has been shown to cause less pain and paresthesias compared to conventional thoracoscopy [13].
A review of the literature reveals that thoracoscopic management of spontaneous pneumothorax is not standardized and that multiple procedures are used in different combinations. A blebectomy or wedge resection of the lung parenchyma (containing the bleb) and apical or total pleurectomy or talc pleurodesis stand out as having the highest chance of preventing recurrences with no increase in morbidity [14]. A combination of a parenchymal resection and pleural procedure appear mandatory to prevent recurrences.
Buttressing of the parenchymal staple line is not standard, although covering the staple line with an absorbable mesh has been shown to eliminate postoperative air leak [15]. Apical pleurectomy is considered equivalent to total pleurectomy with one series showing a lower recurrence rate when a complete pleurectomy is performed. Pleural abrasion, argon beam coagulation of the pleura and pleurodesis using agents other than talc are less successful in preventing a recurrence of the pneumothorax [16-21].
Thoracoscopic pleurectomy and blebectomy has been shown to allow for shorter hospital stay, less pain, and quicker return to the activities of daily life compared to open thoracotomy, which is important in this young, otherwise healthy, patient population [6, 16, 22]. Pleurectomy has been shown to preserve lung function in the long term and does not restrict chest wall mobility [21]. Talc pleurodesis in these patients carries the risk of respiratory failure and additional morbidity should the patient need thoracic surgery later in life. However, a large series in Europe has demonstrated this method to be safe and without long term consequence [14, 17]. Pleurectomy or pleural abrasion and simultaneous placement of talc within the pleural cavity should not be performed due to the systemic absorption of talc and the resultant toxicity.
Morbidity from the thoracoscopic approach is primarily from recurrence (0-5%), bleeding (1-5%), prolonged air leak (1-8%), and chronic chest wall pain (20%) [14, 23, 24]. In the immediate postoperative period, it is probably best to have the chest tubes on water seal rather than suction [25]. Asymptomatic patients who are to undergo surgery should be counseled regarding the chance (20%) of long term chest wall pain and paresthesias [26].
Thoracoscopic pleurectomy and blebectomy is likely to remain the standard of care for spontaneous pneumothorax given its low morbidity, high success rate, and long term durability.
Video 1Video 2Video 3Video 4Video 5Video 6Video 7Video 8Video 9Video 10Video 11References
- Hazelrigg SR, Magee M, Boley TM. Spontaneous Pneumothorax, in Minimal Access Cardiothoracic Surgery,. A.P. Yim, et al. eds, 2000, W. B. Saunders Company, Philadelphia, pp 73-79.
- Beauchamp G, Ouellette D. Spontaneous pneumothorax and pneumomediastinum, in Thoracic Surgery, 2 ed. F.G. Pearson, et al, eds. 2002, Churchill Livingstone: Philadelphia. pp 1202-6.
- Hazelrigg SR, Landreneau RJ, Mack M, et al. Thoracoscopic stapled resection for spontaneous pneumothorax. J Thorac Cardiovasc Surg 1993;105:389-93.
- Liu HP, Yim A, Izzat M, Lin PJ, Chang CH. Thoracoscopic surgery for spontaneous pneumothorax. World J Surg 1999;23:1133-6.
- Naunheim K, Mack M, Hazelrigg SR, et al. Safety and efficacy of video-assisted thoracic surgical techniques for the treatment of spontaneous pneumothorax. J Thorac Cardiovasc Surg 1995;109:1198-1204.
- Sawada S, Watanabe Y, Moriyama S. Video-assisted thoracoscopic surgery for primary spontaneous pneumothorax: evaluation of indications and long-term outcome compared with conservative treatment and open thoracotomy. Chest 2005;127:2226-30.
- Margolis M, Gharagozloo F, Tempesta B, Trachiotis G, Katz N, Alexander P. Video-assisted thoracic surgical treatment of initial spontaneous pneumothorax in young patients. Ann Thorac Surg 2003;76:1661-4.
- Freixinet JL, Canalís E, Juliá G, Rodriguez P, Santana N, de Castro FR. Axillary thoracotomy versus videothoracoscopy for the treatment of primary spontaneous pneumothorax. Ann Thorac Surg 2004;78:417-20.
- Horio H, Nomori H, Fuyuno G, Kobayashi R, Suemasu K. Limited axillary thoracotomy vs video-assisted thoracoscopic surgery for spontaneous pneumothorax. Surg Endosc 1998;12:1155-8.
- Ayed AK. Bilateral video-assisted thoracoscopic surgery for bilateral spontaneous pneumothorax. Chest 2002;122:2234-7.
- Maruyama R, Anai H. Video-assisted thoracoscopic surgery for bilateral spontaneous pneumothorax in supine position: the use of a pillow beneath the back for intercostal space widening. Thorac Cardiovasc Surg 2000;48:370-1.
- Wu YC, Chu Y, Liu YH, Yeh CH, Chen TP, Liu HP. Thoracoscopic ipsilateral approach to contralateral bullous lesion in patients with bilateral spontaneous pneumothorax. Ann Thorac Surg 2003;76:1665-7.
- Jutley RS, Khalil MW, Rocco G. Uniportal vs standard three-port VATS technique for spontaneous pneumothorax: comparison of post-operative pain and residual paraesthesia. Eur J Cardiothorac Surg 2005;28:43-6.
- Tschopp JM, Rami-Porta R, Noppen M, Astou P. Management of spontaneous pneumothorax: state of the art. Eur Respir J 2006;28:637-50.
- Sakamoto K, Takei H, Nishii T, et al. Staple line coverage with absorbable mesh after thoracoscopic bullectomy for spontaneous pneumothorax. Surg Endosc 2004;18:478-81.
- Ben-Nun A, Soudack M, Best LA. Video-assisted thoracoscopic surgery for recurrent spontaneous pneumothorax: the long-term benefit. World J Surg 2006;30:285-90.
- Cardillo G, Carleo F, Giunti R, et al. Videothoracoscopic talc poudrage in primary spontaneous pneumothorax: a single-institution experience in 861 cases. J Thorac Cardiovasc Surg 2006;131:322-8.
- Chang YC, Chen CW, Huang SH, Chen JS. Modified needlescopic video-assisted thoracic surgery for primary spontaneous pneumothorax : the long-term effects of apical pleurectomy versus pleural abrasion. Surg Endosc 2006;20:757-62.
- Czerny M, Salat A, Fleck T, et al. Lung wedge resection improves outcome in stage I primary spontaneous pneumothorax. Ann Thorac Surg 2004;77:1802-5.
- Gossot D, Galetta D, Stern JB, et al. Results of thoracoscopic pleural abrasion for primary spontaneous pneumothorax. Surg Endosc 2004;18:466-71.
- Leo F, Pastorino U, Goldstraw P. Pleurectomy in primary pneumothorax: is extensive pleurectomy necessary? J Cardiovasc Surg (Torino) 2000;41:633-6.
- Santillan-Doherty P, Argote-Greene LM, Guzman-Sanchez M. Thoracoscopic management of primary spontaneous pneumothorax. Am Surg 2006;72:145-9.
- Cardillo G, Facciolo F, Giunti R, et al. Videothoracoscopic treatment of primary spontaneous pneumothorax: a 6-year experience. Ann Thorac Surg 2000;69:357-62.
- Doddolia C, Barlésib F, Fraticellib A, et al. Video-assisted thoracoscopic management of recurrent primary spontaneous pneumothorax after prior talc pleurodesis: a feasible, safe and efficient treatment option. Eur J Cardiothorac Surg 2004;26:889-92.
- Ayed AK. Suction versus water seal after thoracoscopy for primary spontaneous pneumothorax: prospective randomized study. Ann Thorac Surg 2003;75:1593-6.
- Sihoe AD, Au SS, Cheung ML, et al. Incidence of chest wall paresthesia after video-assisted thoracic surgery for primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2004;25:1054-8.



