Patient Presentation
The patient is a thin, 40-year-old smoker who presented to the Emergency Department complaining of chest discomfort and numbness. He noticed a new mass on his left chest wall, which had grown gradually larger over the last 3 months. More recently, he had developed paraesthesia in the skin overlying the mass and had decreased range of motion of his left shoulder from chest tightness. The patient has a history of alcoholism and poor dentition including recurrent dental abscesses, the most recent which was two months and drained itself spontaneously. He states he has had a dry, non-productive cough for the prior year. He reports a recent 15-20 lb. weight loss and a 4-week history of night sweats. His past medical and surgical history is otherwise unremarkable except for childhood asthma. Additional review of systems was unrevealing.
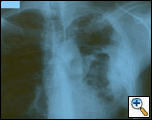 |
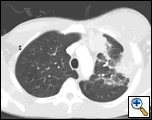
| Figure 1: PA chest film reveals a left pleural-based mass with pleural thickening and upper lobe scarring. |
On physical examination, the patient was afebrile with stable vital signs. He was a thin male who appeared older than his stated age. Head and neck exam revealed poor dentition without any palpable cervical or supraclavicular lymphadenopathy. There was a left chest wall mass approximately 6 cm in diameter surrounding the left nipple, which was firm, non-mobile and non-tender to palpation. There was no skin discoloration, erythema, fluctuance, or draining sinuses noted. The patient had full passive range of motion of his left shoulder. The lung exam was clear bilaterally with no rales, ronchi or significant wheezing. His cardiovascular and remaining exam was without gross abnormality. His chemistry lab values were within the normal range. The patient has a white blood cell count of 9,800, a normal differential, and his HIV serologies were negative.
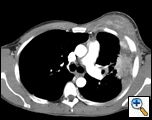
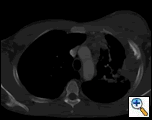
PA and lateral chest film revealed patchy consolidation of the left lung with a large pleural-based mass, left apical pleural thickening, and right upper lobe scarring (Figure 1). Helical CT scan of the chest, abdomen, and pelvis with contrast demonstrates a large, 10.8 x 4.1 cm left pleural-based mass extending into the chest wall and left breast (Figure 2). There was periosteal thickening and irregularity of adjacent ribs. Multiple enlarged mediastinal, left hilar, and supraclavicular nodes were noted, the largest 1.5 x 2.6 cm in the AP window or station five region. There was patchy consolidation of the left and right upper lobes. An MRI of the thorax with contrast was obtained and confirmed the presence of left supraclavicular adenopathy and an enhancing mass with extension into the chest wall involving the ribs, infiltration of the pectoralis muscle, and extension into the left lung parenchyma. The main differential diagnoses at this time were neoplasm (sarcoma, advanced lung cancer, mesothelioma, lymphoma, osteosarcoma).
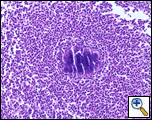
A Tru-cut needle biopsy was performed at bedside which revealed nonspecific pathologic findings, but a repeat with increased sampling revealed acute and chronic inflammation with granulation tissue containing Actinomyces confirmed by periodic acid shift (PAS), Gomori’s methamine silver (GMS), and modified Gram stains (Figure 3). Treatment began with intravenous Penicillin G (4 million units every 4 hours for a 6 week course) which will be followed by oral amoxicillin for 6-12 months. A repeat CT scan was planned in 4 weeks, as actinomycosis is known to colonize malignant tissue. The patient, at three month follow up, was doing well from a pulmonary standpoint and had regression of his chest wall mass.
Discussion
Actinomycosis is a chronic, suppurative granulomatous infection caused by Actinomyces species, most commonly A. israelii. Many cases of actinomycosis are actually polymicrobial and spread by direct extension in to adjacent tissues [1]. The first case report in humans was in 1857, followed by the first case report of thoracic actinomycosis in 1882 [6]. The most common presentation (>50%) of actinomycosis is cervicofacial, commonly presenting as a jaw mass, with thoracic actinomycosis accounting for between 15-50% of cases depending on the series [1,6]. The disease occurs in a 3:1 male to female ratio, possibly because of poorer oral hygiene and a higher incidence of facial trauma in males [1]. There is also a bimodal age distribution in occurrence: 11-20 and the 4th-5th decades of life, where this patient fits [1]. Patients with underlying diseases are predisposed to infection, but it can occur in otherwise healthy individuals. Interestingly, there does not seem to be any increase in patients who are immune compromised including those with HIV, organ transplant, or on steroids [6]. In the United Kingdom, patients with COPD, bronchitis, and alcoholics are predisposed to actinomycosis, while in Japan an association is found with dental caries (19%), diabetes (13%), and pyorrhea alveolaris (6%) [6,4]. The most common presentation of pulmonary actinomycosis in the UK is cough, fever, and chest pain. In a review of 95 cases in Japan, the most common presentations were: hemoptysis (42%), cough or sputum (37%), fever (28%) [1,4]. Advanced or disseminated disease can be associated with weight loss, malaise, high fever, and draining sinuses.
Actinomycosis can be found in the flora of the oropharynx, especially in dental plaque, and in the GI tract. Thus aspiration and aerosolization have been proposed as mechanisms of pulmonary actinomycosis [1,6]. This mechanism could correlate with the higher incidence in alcoholics and in patients with poor oral hygiene, like the patient presented here. This etiology is also consistent with the frequent finding of lower lobe and peripheral lung involvement of pulmonary actinomycosis. However, other etiologies like hematogenous spread of septic emboli from vessel erosion have been proposed since apparently normal individuals can be afflicted with pulmonary actinomycosis and upper lobe radiologic abnormalities have been noted with some frequency [1,2,4]. Overall, actinomycosis is quite rare and decreasing in incidence and virulence, perhaps due to better oral hygiene and more prevalent use of antibiotics for treating other conditions [2,6].
The difficulty in diagnosis lies in the rarity of the disease, its radiologic resemblance to malignant disease, and the difficulty in identifying or culturing the organism (<50% successful), since it is very fastidious and requires special staining with methamine silver (GMS) or modified Gram stain [1]. The observation of “branching filaments” is insufficient to distinguish between colonization and infection, but the presence of “sulfur granules” which are actually colonies of Actinomyces, are suggestive of infective actinomycosis [1]. Laboratory findings are nonspecific; a mild leukocytosis and elevated inflammatory markers including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) may be seen [6]. Common radiographic findings of thoracic actinomycosis include wavy periostitis, pulmonary fibrosis, cavitation, and pleural thickening, and pulmonary osteoarthropathy [2]. Many of these features were seen radiographically in the current case and can be confused with malignancy. A normal bronchus seen entering a mass lesion can be helpful in distinguishing actinomycosis from malignancy, since a normal bronchus is almost never seen with a large tumor [2]. Fine needle aspiration (FNA) or Tru-cut biopsy can be used for tissue diagnosis, but has a high false negative rate as seen by the difficulty in obtaining an adequate sample in the current case. Video assisted thoracoscopy (VATS) may be particularly useful for the diagnosis of peripheral lung or thoracic actinomycosis [4]. It offers the advantages of direct visualization and the ability take larger tissue samples [4]. Unfortunately, most of the diagnoses of thoracic or pulmonary actinomycosis are still made at thoracotomy, including over half of the cases in Japan and Taiwan [3,4].
The first line treatment for actinomycosis is medical with extended courses of intravenous penicillin for 2-6 weeks followed by oral penicillin or amoxicillin for 6-12 months. Doxycycline is a can be used as a substitute in penicillin allergic patients [1,6]. Surgery has a role in managing complications of actinomycosis, including abscess, empyema, fistula, and hemoptysis. Hemoptysis due to actinomycosis has been found to have a 36.4% rate of re-bleeding within six months of hospital discharge for patients who were treated medically, and a 50% mortality among those patients who re-bleed [5]. Early treatment leads to an excellent outcome with low mortality, but overall, pulmonary actinomycosis has a poorer prognosis than disease in other locations [6].
References
- Bennhoff DF. Actinomycosis: diagnostic and therapeutic considerations and a review of 32 cases. Laryngoscope 1984;94:1198-1217.
- Flynn MW, Felson B. The roentgen manifestations of thoracic actinomycosis.
Am J Roentgenol Radium Ther Nucl Med. 1970;110:707-16. - Hsieh MJ, Lui HP, Chang JP, Chang CH. Thoracic actinomycosis. Chest 1993;104:366-370.
- Kobashi Y, Yoshida K, Miyashita N, Niki Y, Matsushima T. Thoracic actinomycosis with mainly pleural involvement. J Infect Chemother 2004;10:172-177.
- Lu MS, Liu HP, Yeh CH, et al. The role of surgery in hemoptysis caused by thoracic actinomycosis: A forgotten disease. Eur J of Cardiothorac Surg 2003; 24:694-698.
- Mabezza GF, MacFarlane J. Pulmonary Actinomycosis. Eur Respir J 2003;21:545-551.