ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
VATS Major Pulmonary Resection
Patient Selection
VATS for major lung resection represents a new approach and not a new procedure. Therefore, the indications for VATS major lung resections remain the same as for conventional approaches to resection. In our institution, close to 80% of lung resections are for early, primary lung cancer, with the remaining for metastatic lung cancer and benign diseases like localized bronchiectasis or multi-drug resistant tuberculosis [1]. For primary lung cancer, preresectional staging (including mediastinoscopy) remains a critically important step in cancer management and should be strictly adhered to.
There are few absolute contraindications that are specifically applicable to VATS major resections. Apart from the inability to tolerate single lung ventilation, all of them are anatomical considerations. We do not recommend VATS for tumors larger than 4 cm, not primarily because of technical difficulties, but because ribs have to be excessively spread to retrieve the specimen and this tends to negate the benefit of minimal access surgery. True pleural symphysis that leads to abandonment of the VATS approach is uncommon in our experience. Once a space is created when the correct plane in the pleural space is entered, endoscopic adhesiolysis can proceed quickly and safely. VATS has the advantage over conventional thoracotomy in visualizing details the apex and base of the hemithorax with high resolution. Redo-VATS surgery has been reported, and prior surgery is no longer considered an absolute contraindication to VATS resection [2]. Fused fissures present a technical challenge to VATS lobectomy. However, with experience and proper intraoperative planning, successful lobectomy can be accomplished. The fused fissure should be divided last following the pulmonary vasculatures and the bronchus.
Operative Steps
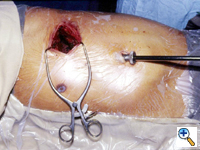
The surgeon stands facing the patient who is in the lateral decubitus position under general anesthesia with selective one lung ventilation [3]. We prefer to flex the operating table to open up the intercostal spaces for instrument maneuvering (Figure 1). The thoracoscope is normally placed in the seventh or eighth intercostal space over the mid to anterior axillary line, depending on the body build of the patient and the location of the pathology. From this position, a panoramic view of the hemithorax can be obtained. The basic principle is to align the thoracoscope, the pathology and the television monitor. This allows the surgeon to look straight ahead during the operation and provides the best ergonomic position for the surgeon [4].
If there is no contraindication to proceed, a minithoracotomy (generally 6 to 8 cm in length) is placed over the fourth intercostal space in the anterolateral chest (Figure 2). In females, the skin incision can be made (if the anatomy allows) in the inframammary fold for cosmesis. The location of this wound usually means only a small portion, if any, of the latissimus dorsi muscle needs to be divided. The serratus anterior muscle is split along the direction of its fibers. Division of the intercostal muscles permits access into the pleural cavity. The placement of the minithoracotomy in this position provides direct access to the hilum, and because the anterior intercostal space is wider than the posterior space, it facilitates later retrieval of the specimen. We do not use rib spreader, only a soft tissue retractor for the minithoracotomy wound.
A sponge holding forceps is introduced through a separate tiny incision in the seventh or eighth intercostal space in the posterior axillary line. With two sponge holding forceps, one through the anterior minithoracotomy and the other through the posterior wound, the collapsed lung can be moved around and a thorough assessment made of the location and extent of the primary lesion. This technique also permits a search for associated pathology (satellite nodules; mediastinal or hilar lymphadenopathy which could be missed even by high resolution contrast chest CT).
Contrary to laparoscopic surgery, which generally does not allow digital palpation (because of the need to use valved ports to sustain carbon dioxide insufflation), digital palpation through the minithoracotomy, in addition to instrument palpation during VATS exploration, can provide valuable information to the surgeon and is encouraged. By bringing the lung towards the palpating finger placed through different sites, a large portion of the lung surface can be palpated. This is important as small nodules (< 0.5 cm) which are not subpleurally located would almost certainly go undetected by thoracoscopic examination alone.
Conventional Metzenbaum scissors and DeBakey forceps are introduced through the minithoracotomy wound for sharp dissection of the hilum. The sponge holding forceps from the posterior wound is used to provide appropriate traction and to position the lobes so that the hilum can be easily accessed through the minithoracotomy. If there are adhesions of the lung to the chest wall or mediastinum, these should be taken down before starting the hilar dissection as it is essential to be able to freely move the lobes around.
If the interlobar fissure is complete or nearly complete, sharp incision of the visceral pleura and blunt dissection using a dental pledget mounted on a conventional curved clamp allow easy identification of the pulmonary artery. If the fissure is not complete, we find a monopolar diathermy forceps (Olsen Electrosurgical, Inc., Concord, CA) at a low setting to be useful for hemostasis when a layer of lung parenchyma has to be divided to access the interlobar vessels.
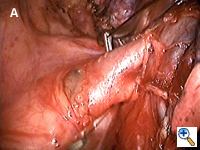
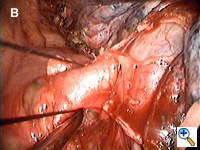
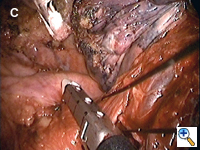
Dissecting around a pulmonary vessel is basically the same as in conventional, open surgery. We use a right-angle Mixter clamp to go around a vessel, and then loop it with a heavy silk ligature. With gentle traction on the suture, a dental pledget is used to gently dissect the undersurface of the vessel. A mechanical stapler (Endo-GIA 30V; Autosuture, United States Surgical, Norwalk, CT) is then introduced through one of the other two ports (depending on the alignment), but usually the camera port (with the thoracoscope repositioned to view through the minithoracotomy wound) to staple-transect the vessel. Appropriate traction (using a sponge holding forceps from the other port) is crucial in aligning the vessel with the stapler for transection (Figure 3). In recent years, we have been using more frequently ligation of pulmonary arterial branches with extracorporeal knots instead of mechanical staplers mainly to reduce cost.[5] We tend not to use many endoclips for small pulmonary branches, as their presence could interfere with the subsequent use and functioning of the endostaplers.
Lewis and his colleagues have accumulated extensive experience on simultaneously staple-transecting the pulmonary vasculature and lobar bronchus with excellent results.[6] The author has modified and adopted this technique for lower lobectomy (for bronchiectasis) on a couple of occasions, during which individual isolation of the pulmonary artery and bronchus to the lower lobe proved to be technically difficult or even hazardous. On both occasions, the inferior vein was taken separately.
The lobar bronchus is divided with a linear mechanical stapler with a built in blade for transection (Endo-GIA 30 with closed staple height of 1.5 mm; Autosuture, USC, Norwalk, CT). The mainstem bronchus requires use of a different stapler that necessitates manual transection (Roticulator 30 with closed staple height of 2 mm; Autosuture, USC, Norwalk, CT). The integrity of the bronchial stump is then tested to 35 cm of airway pressure in the usual manner underwater.
Release of the pulmonary ligament is required following upper and/or middle lobectomies. Although this is usually a straightforward maneuver, the positions of the ports and minithoracotomy mean that paradoxical motion would be difficult to avoid. Paradoxical motion is generated when the camera and instruments are facing each other. We have found that by turning the camera 180 degrees, a normal spatial relationship is restored for the operator.[7] This simple maneuver allows the surgeon to use the camera and existing ports to his/her best ergonomic advantage.
We do not perform VATS mediastinal lymphadenectomy. If a complete lymph node dissection is considered necessary, it should be done through a thoracotomy. There are surgeons who advocate routine VATS lymphadenectomy, but just like open surgery, their results have not been shown to be superior to those who perform lymph node sampling.[8]
A wound protector should be used to avoid any potential implantation of tumor cells in the wound. When the primary tumor is less than 4 cm, we generally have found that a mechanical rib spreader is not necessary for retrieval of the resected lobe, bi-lobe or even the entire lung (although some manual rib retraction may be needed at the time of retrieval).
The entire hemithorax is then copiously irrigated with warm saline. If there is any significant parenchymal leak, endoscopic suturing can be done through the mini-thoracotomy.
The author describes his own technique here, but the readers are reminded that other variations in technique exist for this procedure.[9]
Preference Card
- Conventional thoraic instruments for hilar disection.
- Sponge Holding forceps for handling the lung.
- A thoracotomy tray should be at hand.
Tips & Pitfalls
- VATS major resection currently is not for every patient. The surgeon should have accumulated a fairly large experience on VATS before attempting endoscopic major resection.
- Although manual (and bimanual) palpation is not possible with VATS, experienced VATS surgeons have all learned to feel with the tips of their instruments. Long (and hence heavier) instruments are generally not necessary for hilar dissection and would only decrease the sensitivity of the essential tactile feedback.
- Conventional tools are familiar to the surgeon and shorten the time needed to acquire this technique.
- The inherent decrease in tactile information due to the lack of manual palpation during VATS is compensated by the enhancement of visual information - the monitors provide a magnified view of the operative field with high resolution for details. Subtle displacement of a structure during dissection (which is usually not noticeable in conventional open surgery) provides important clues to an experienced VATS surgeon.
Results
Major complications from VATS resections are relatively uncommon. Persistent airleak beyond seven days was the commonest morbidity in earlier experience. [10] This was almost certainly related to hilar dissection when the fissures were incomplete. However, like most technical issues, this improved with experience.
Tumor implantation following VATS has been reported [11]. However, this is relatively uncommon and could be further minimized by gentle handling of tissue, routine use of a wound protector and copious irrigation of the hemithorax prior to closure.
One of the most dreaded complications for surgeons is massive bleeding from pulmonary vessels. Both Walker and the author have reported mechanical failure of the staplers that resulted in massive bleeding [12,13]. This can be controlled by pressing on the bleeder with a sponge stick and conversion to a thoracotomy. It should be pointed out that these are anecdotal cases, and the mechanical staplers available now are generally very reliable. Having now acquired the skill and experience in endoscopic suturing, we feel comfortable to use this technique in the unlikely event of minor to moderate bleeding from the pulmonary vasculature, and hence avoid the need for a conversion.
VATS major resection has now matured to become an established, alternative approach to conventional open surgery for selected patients. The technique has been shown to be safe and there are documental advantage over the conventional approach in terms of less postoperative pain, better preservation of early postoperative lung function, and earlier return to work. Older and sicker patients who may otherwise not be suitable candidates for open resection can be recruited for VATS major resection. The intermediate and long term survival for early lung cancer patients who underwent VATS resection appears at least as good, if not superior to, open surgery [3]. This may be related to better preservation of the body immune function following VATS [14].
References
- Yim APC, Ko KM, Ma CC. Thoracoscopic lobectomy for benign disease. Chest 1996; 109:554-556.
- Yim APC, Liu HP, Hazelrigg SR, et al. Thoracoscopic operations on reoperated chests. Ann Thorac Surg 1998; 65:328-330.
- Yim APC. VATS major pulmonary resection revisited - controversies, techniques and results. Ann Thorac Surg 2002; 74:615-23.
- Landreneau RJ, Mack MJ, Hazelrigg SR, et al. Video-assisted thoracic surgery: Basic technical concepts and intercostals approach strategies. Ann Thorac Surg 1992; 54:800-807.
- Yim APC, Lee TWC. 'Home-made' knot pusher for extracorporeal ties. Aust NZ J Surg 1995; 65: 510-511.
- Lewis RJ, Caccavale RJ, Boncage JP, Widmann MD. Video-assisted thoracic surgical non-rib spreading simultaneously stapled lobectomy: a more patient-friendly oncologic resection. Chest 1999; 116:1119-24.
- Yim APC, Izzat MB, Lee TW. Counteracting paradoxical motion in videoendoscopic operations. Ann Thorac Surg 1998; 66:965.
- Kaseda S, Aoki T, Hangai N. Video-assisted thoracic surgery (VATS) lobectomy: the Japanese experience. Semin Thorac Cardiovasc Surg 1998; 10:300-304.
- Yim APC, Landreneau RJ, Izzat MB, Fung ALK. Is video-assisted thoracoscopic lobectomy a unified approach? Ann Thorac Surg 1998; 66:1155-58.
- Yim APC, Ko KM, Chau WS, et al: Video assisted thoracoscopic anatomic lung resections. The initial Hong Kong experience. Chest 1996; 109: 13-17.
- Downey RJ, McCormick P, LoCicero J, et al: Dissemination of malignant tumors after video-assisted thoracic surgery: A report of twenty-one cases. J Thorac Cardiovasc Surg 1996; 111:954-960.
- Craig SR, Walker WS: Potential complications of vascular stapling in thoracoscopic pulmonary resection. Ann Thorac Surg 1995; 59:736-737.
- Yim APC, Ho JKS: Malfunctioning of vascular staple cutter during thoracoscopic lobectomy. J Thorac Cardiovasc Surg 1995; 109:1252.
- Yim APC. Wan S, Lee TW, Arifi AA. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000; 70:243-7.