ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Video Assisted Thoracoscopic (VATS) Thymectomy
Agasthian T. Video Assisted Thoracoscopic (VATS) Thymectomy. November 2022. doi:10.25373/ctsnet.21528777
Introduction
A variety of approaches for surgical management of myasthenia gravis and thymoma have been described. There is a growing experience with VATS approaches to the management of each of these conditions. One approach to VATS thymectomy is described in this article and the accompanying videos.
Patient Selection
a. Nonthymomatous myasthenia gravis
b. Small (< 2cm) intrathymic thymoma
c. Large well encapsulated thymoma (preferably <5 cm)
d. Minimally invasive thymoma
Operative Procedure
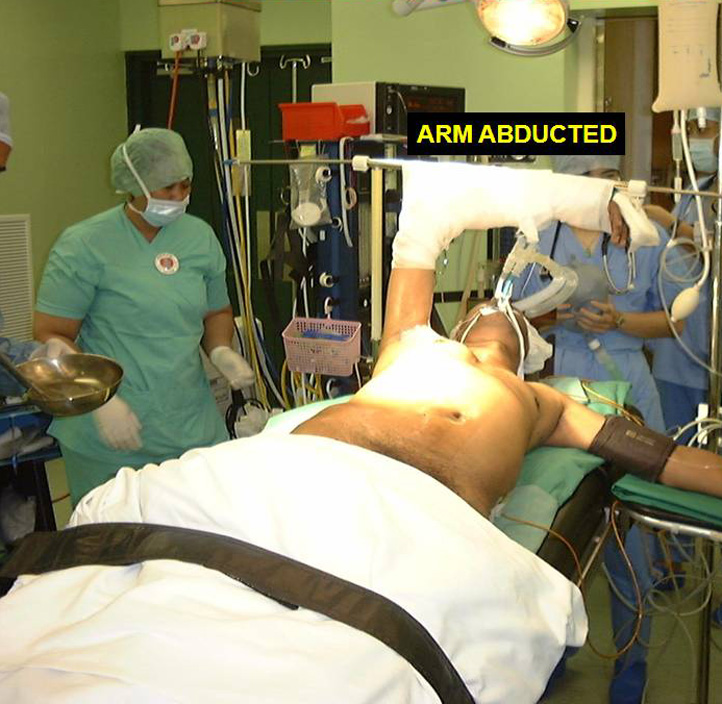
The patient is positioned in a 30 degree semi-supine position with a roll placed under the shoulder, and the ipsilateral arm held abducted over a padded L-screen for exposure of the axilla (Figure 1). The right side is the preferred approach for non thymomatous myasthenia gravis, especially in small females, as there is more room for manipulation with the heart out of the way. The left sided approach is used whenever a thymoma is located on that side. Lung isolation is obtained with a double lumen endotracheal tube supplemented with initial carbon dioxide insufflation (5-8mmHg pressure at 4L/min flow rate) for rapid initial collapse of the lung. The carbon dioxide insufflation is discontinued once the lung is adequately deflated.
Figure 1. Position for right VATS thymectomy.
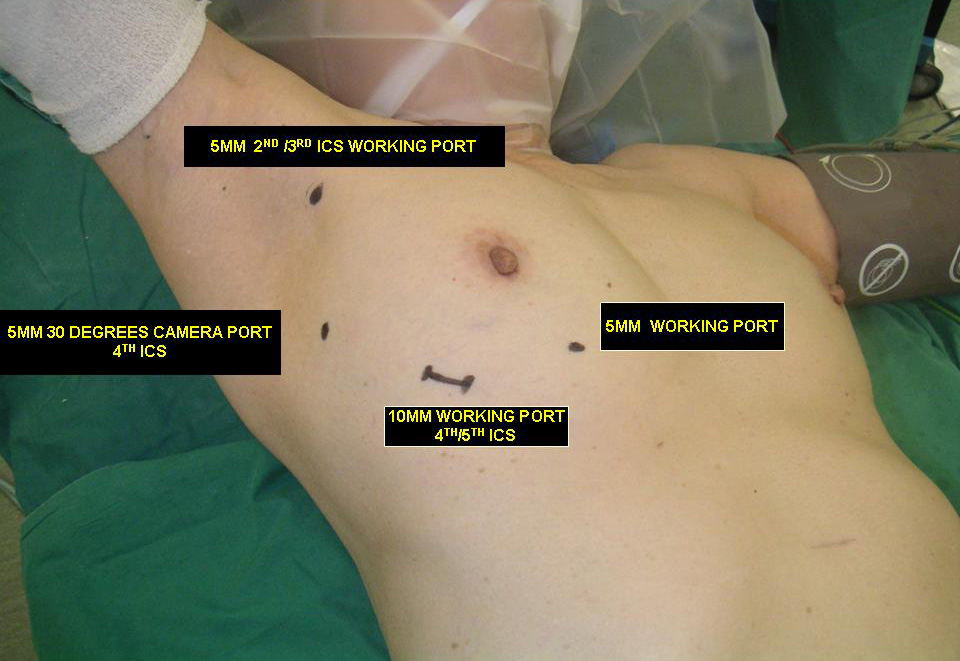
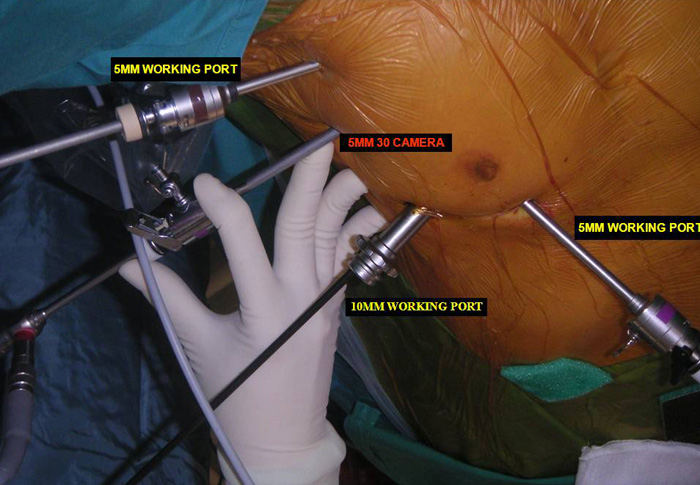
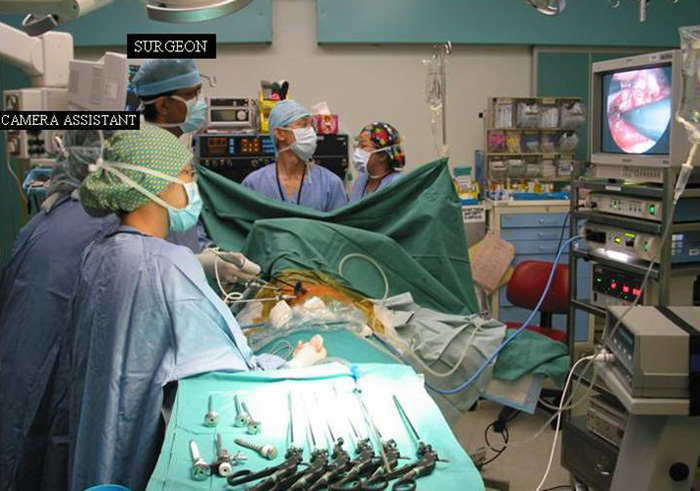
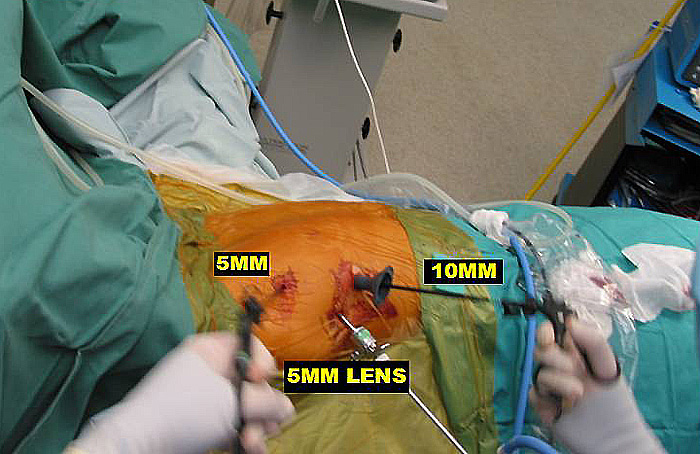
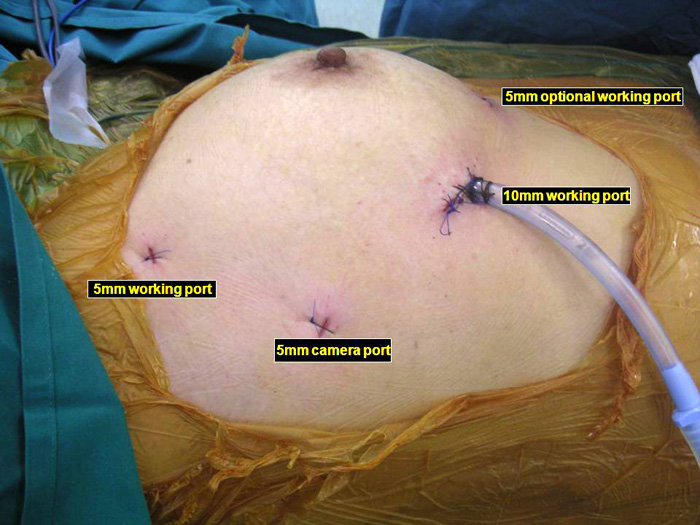
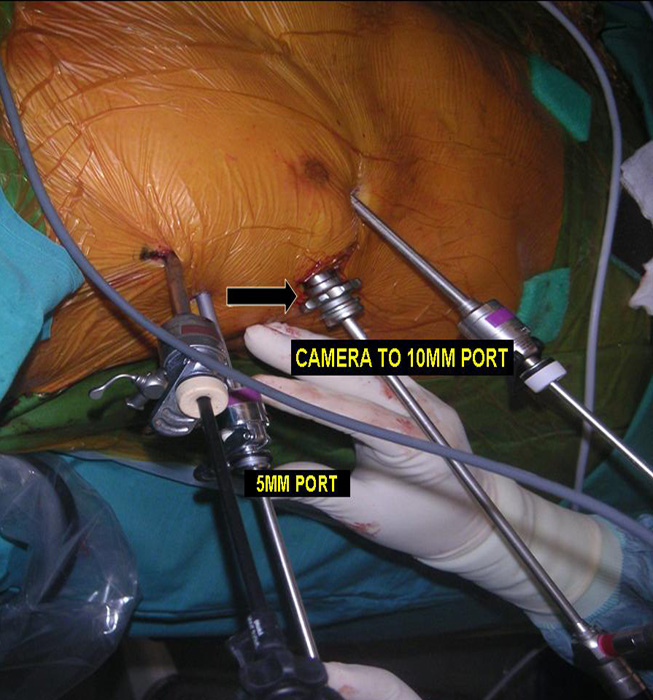
Three 5mm and one 10mm ports are inserted. A 30 degree angled camera is placed in the most lateral 5mm port, the other ports being working ports. A 5mm 30 degree camera is used and the medial most 5mm port is used for retraction by the 2nd assistant (Figures 2, 3). The surgeon and camera man stand on the same side while the scrub technician and 2nd assistant stand on the opposite side (Figures 4, 5). The specimen is removed through the 10mm port through which a chest tube is placed at end of procedure (Figures 6, 7).

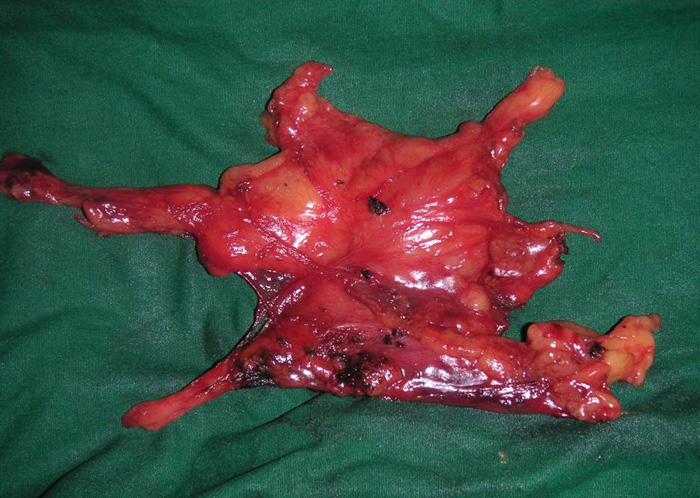
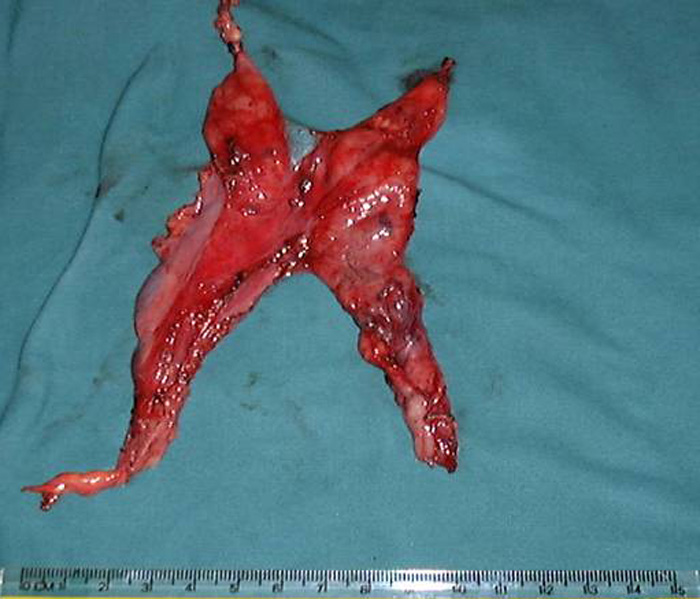
In patients with myasthenia gravis, complete radical thymectomy is achieved by en-bloc removal of the thymus, perithymic fat and tissue from the thoracic inlet to the diaphragm, and from phrenic nerve to phrenic nerve. All horns can be easily dissected right up into the neck and delivered into the chest cavity (Video 1). Accessory horns usually under the innominate vein should be routinely looked for and not missed (Figure 8 and Video 2). The thymus is dissected right up to the opposite phrenic nerve, which is identified by shifting the camera to the 10mm medial port (Figure 9) and by opening the opposite pleura with retraction of the lung by the 2nd assistant through the medial most 5mm port (Video 3). Injury to the phrenic nerves is prevented by using bipolar or other energy sources. Dissection and hemostasis of the thymic vessels is facilitated by use of the ultrasonic harmonic scalpel (Video 4). Radicality and completeness is further ensured at time of surgery by anatomical inspection the resected gland for completeness and also inspection and remove of residual fat and ectopic thymic tissue from the thymic bed and possible ectopic sites (Figure 10).

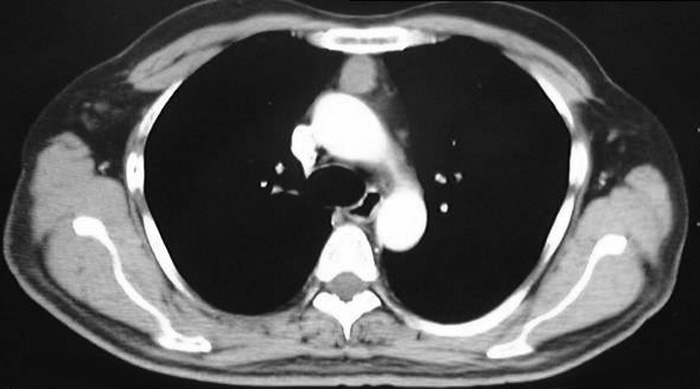
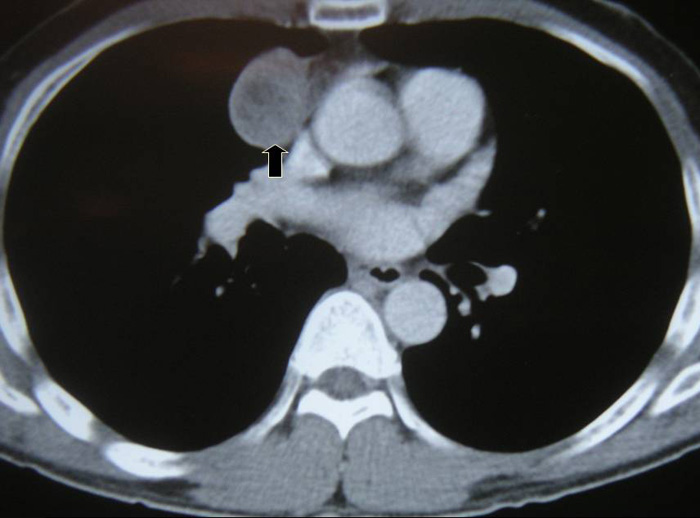
VATS thymectomy for thymoma is generally confined to small intrathymic and encapsulated tumours (Figures 11 and 12, Video 5) as there are oncological concerns of possible breach of the tumour capsule with the risk of tumour seeding. When performing VATS thymomectomy a modified no touch technique is used to minimize the risk of capsular breakage and tumour seeding, which are:
a.Only well encapsulated tumours screened on CT scan thorax are ideal for VATS.
b.The tumour should be approached from the side of the tumour so that dissection is done under direct vision.
c.The tumour should be dissected last using a no touch technique. The non tumourous part of the gland is always dissected first and used for grasping and traction when dissecting the tumour last, thus avoiding touching or grasping the tumour capsule.
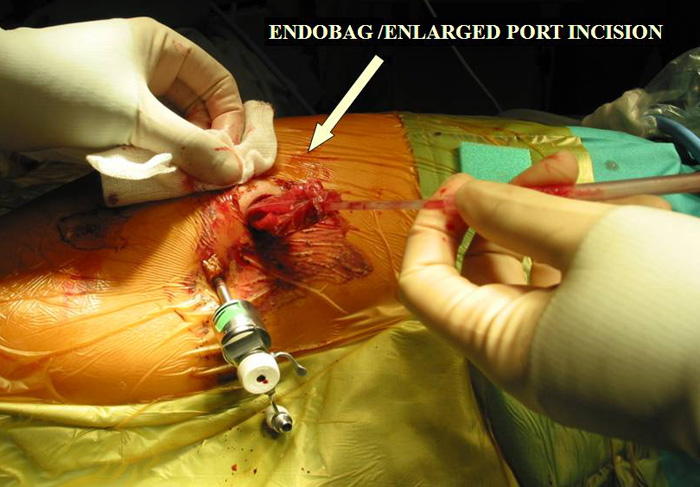
d.Tumour is removed via an Endobag by enlarging the 10mm incision.

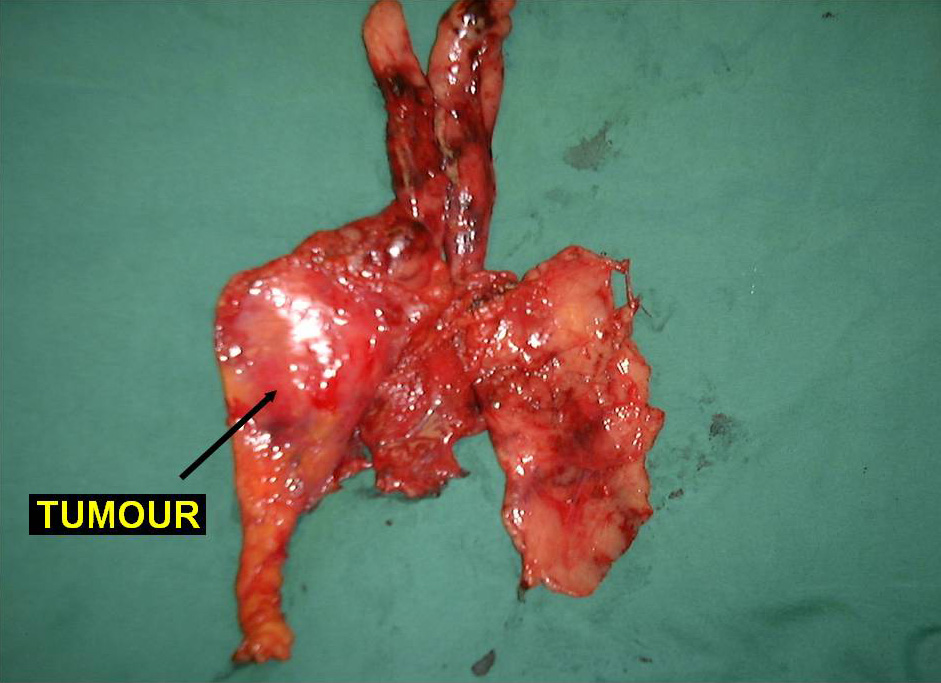
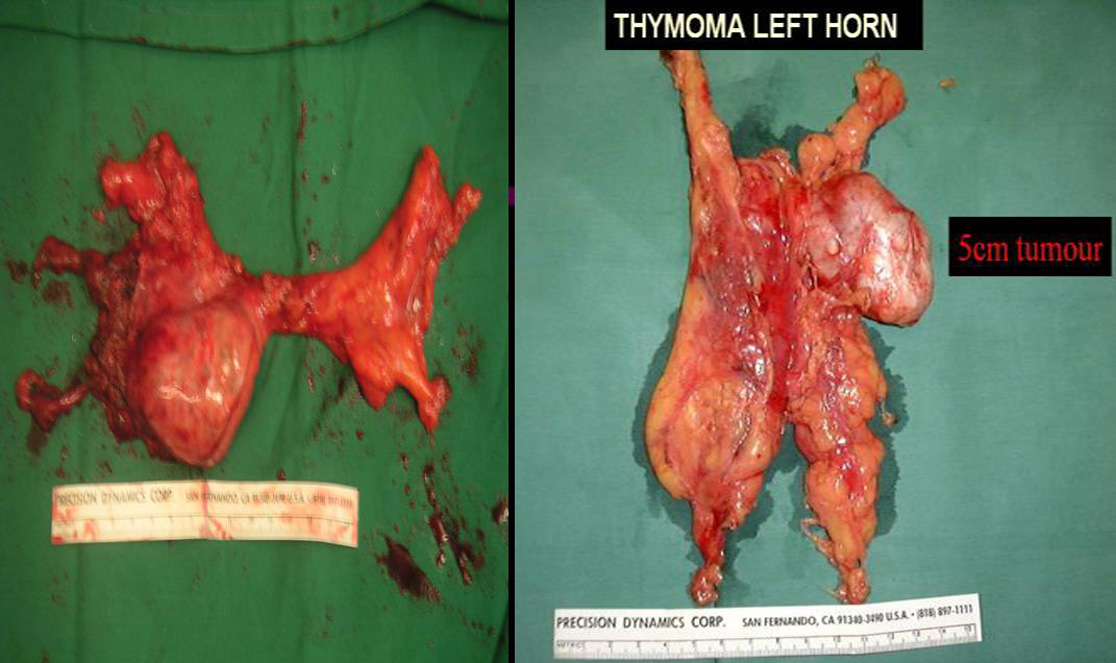
Larger well-encapsulated tumours, preferably less than 5cm, are also suitable for VATS provided the above surgical oncological principles are strictly adhered to (Figures 13 and 14, Video 6). Minimally invasive thymomas are tumours detected to have limited invasion of a segment of lung, pleura, phrenic nerve or pericardium at the time of VATS. The above described oncological principles are adopted here where the non-tumorous part of the gland is mobilized first followed by en bloc removal of tumour together with a cuff of involved pericardium, lung or phrenic nerve. In invasive thymomas division of the involved phrenic nerve and a wedge of lung are carried out early so it can be used for traction and grasping when dissecting the tumor last. This is done by creating a passage between the lung (usually the anterior segment of upper lobe) and tumour. An endostapler is then introduced through the 10mm port and the involved lung is wedged by passing the stapler through the created passage. The tumor is always dissected last, especially if it invades the pericardium, because on opening the pericardium the pulsating heart makes subsequent dissection of the gland and tumor difficult and hazardous (Video 7). Dissecting the tumor last also minimizes tumor handling and manipulation. If the tumor is dissected first, its weight will obstruct the surgical field, making subsequent dissection of the rest of the gland difficult and oncologically hazardous (Video 8).

Preference Card
- 5mm 30 Degree Telescope
- Two 5mm Maryland Dissectors
- 5mm Endoscopic Sucker
- 5mm Endoscopic Energy Source (ultrasonic shears)
- 10mm Endoscopic Retrieval Bag
Tips and Pitfalls
- Gaining experience with VATS thymectomy should be gradual starting with non-thymomatous glands followed by small thymomas, larger tumours and finally invasive tumours.
- In myasthenia gravis completeness of thymectomy is crucial as it will affect long-term clinical outcomes. This is ensured at time of surgery by anatomical inspection of the resected gland for completeness and of the thymic bed for perithymic fat and ectopic thymic tissue. Always look for accessory horns, especially under the innominate vein, where they can be missed easily (Figure 6 and Video 8).
- Identification of both phrenic nerves should be routine and allows safe complete thymectomy to be performed. Thermal injury to the nerves can be minimized by using a safe energy source. Energy sources other than monopolar cautery also allow for quick dissection and safe coagulation of the thymic vessels.
- The superior horns are usually dissected from the innominate vein first, followed by dissection of the body, and then the inferior horns of the thymus are sequentially mobilized. This is to prevent the weight of the gland from obstructing the surgical field of vision and making subsequent dissection from the innominate vein often difficult. This is especially important in thymomas where excessive manipulation should be avoided and the initially dissected horns can be used for traction when mobilizing the tumour last without risking injury to the tumour capsule.
- Though most surgeons use 3 ports, routine retraction through the additional 4th medial most 5mm port has helped increase exposure and quicken the surgery especially for large hyperplastic glands with abundant perithymic fat.
- The semi supine position allows for quick conversion to a sternotomy if necessary.
- When both pleural cavities are opened 1 chest tube suffices, as both pleural spaces function as one compartment.
Results
From 1998 to 2011, 155 VATS thymectomies were performed: 80 for non thymomatous myasthenia gravis, 40 for thymomas associated with myasthenia gravis, and 38 for thymomas. 93% were approached from the right side. Mean age was 44 years (range 12-83 years), mean duration of surgery was 143 minutes, and mean duration of hospital stay was 5 days. There was no hospital mortality and operative morbidity was 13%. Mean follow up was 5.5 years (range 2-11 yrs).
Among myasthenic patients 93.4% had improvement in symptoms with 80% being asymptomatic and 21.3% in complete stable remission without medications. There was no statistical significance in remission rates between thymomatous and non- thymomatous myasthenia gravis.
The mean diameter of thymomas was 5cm (range 1-9 cm). A right sided approach was used in 79.3%. 30 patients were in Masoaka stage I, 34 in stage II, 12 in stage III and 1 in stage IV. The 13 stage III and IV patients were found to be locally invasive only at time of VATS. The mean diameter of minimally invasive thymomas was 34mm (range 23-55 mm). These cases had limited resection of involved structures (perithymic fat, cuff of pericardium, phrenic nerve and wedge of lung were removed en bloc with the tumour and thymus gland). To date all patients are alive with 2 local recurrences.
Video 1. Dissection of the thymus into the neck
Video 2. Delivery of superior and accessory horns from the neck into the mediastinum.
Video 3. Dissection of left lobe of thymus from the opposite phrenic nerve under direct vision.
Video 4. Dissection and division of thymic vessels with ultrasonic harmonic scapel.
Video 5. VATS thymectomy for small thymomas(<2cm)
Video 6. VATS thymectomy for large well encapsulated thymomas.
Video 7. VATS thymectomy for invasive thymomas.
Video 8. Wrong VATS thymectomy technique where the tumor is dissected first.
References
1.Vyas S, Agasthian T, Goh MH, Shankar S. Thoracoscopic thymectomy in a previous sternotomy. Asian Cardiovasc Thorac Ann 2006; 14(6):e108-10.
2. Soon JL, Agasthian T. Harmonic scalpel in video-assisted thoracoscopic thymic resections. Asian Cardiovasc Thorac Ann 2008; 16(5):366-9.
3. Agasthian T, Lin SJ. Clinical outcomes of VATS for thymomas. Interact Cardio Vasc Thorac Surgery 2008:7: abstract 021, suppl 3 to Vol 7.
4. Agasthian T, Lin SJ. Clinical outcome of video-assisted thymectomy for myasthenia gravis and thymoma. Asian Cardiovasc Thorac Ann 2010; 18(3):234-9.
5. Agasthian T. Can invasive thymomas be resected by video assisted thoracoscopic surgery? Asian Cardiovasc Thorac Ann 2011; 19:225-227.
Disclaimer
The information and views presented on CTSNet.org represent the views of the authors and contributors of the material and not of CTSNet. Please review our full disclaimer page here.




Comments