ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
The Cardiovascular Hybrid OR-Clinical & Technical Considerations
Index
Index
» Introduction
» Usage of hybrid OR
» Basics of the hybrid room
» Imaging equipment
» Conclusions
» References
Disclosure
All three authors are working for Siemens Healthcare which is offering angiography systems for hybrid ORs
Introduction
Recent developments in cardiac care have led to new therapies integrating surgical procedures with skin incisions and interventions, e.g. transcatheter techniques with the puncture of a vessel. To allow these procedures, integrated operating rooms have to be installed. These hybrid operating rooms need in excess of surgical equipment high-end imaging equipment equivalent to the angiography devices used in interventional radiography and cardiology. Imaging devices have been used in an operating theatre for a long time. Mobile C-arms, ultrasound, and endoscopy are standard of care for many operations. However, complex transcatheter techniques demand high powered equipment to visualize thin guide wires, quantify small vessel diameters, and evaluate delicate anastomoses. Because of their size and complexity these integrated endovascular suites or Hybrid ORs require special considerations, planning, and design as well as new skills to be learned by the team.
Usage of a hybrid operating room
Before a hybrid operating room is planned, all stakeholders in the hospital should be identified and a detailed plan of room usage developed. These high tech rooms are too costly for part time use. However, once such a facility is constructed the demand for it is increasing, because of growing indications and increased referrals (20).
Pediatric hybrid operations, hybrid coronary revascularization, transcatheter valve replacement and repair, and stent graft placement in the thoracic aorta are just some of the new developments that are ideally performed in a hybrid operating room. Although hybrid therapies were probably first developed in close team work of pediatric cardiology and pediatric cardiac surgery, currently the strongest driver for hybrid therapies is transcatheter replacement of the stenotic aortic valve.
Hybrid therapy for congenital heart disease
Although surgery remains the treatment of choice for many congenital cardiac malformations, interventional cardiology approaches are increasingly being used in simple and even complex lesions. The percutaneous approach can be challenging due to low patient weight or poor vascular access, induced rhythm disturbances and hemodynamic compromise (2). Difficult and complex anatomy as in double-outlet right ventricle or transposition of the great arteries, or acute turns or kinks in the pulmonary arteries of tetralogy of Fallot patients can make percutaneous procedures challenging if not impossible (19). On the other hand, surgery also has its limitation. Examples are operative closure of multiple apical muscular ventricular septal defects, adequate and lasting relief of peripheral pulmonic stenosis, or management of a previously implanted stenotic stent. Combining interventions and surgery into a single therapeutic procedure leads to reduction of complexity, cardiopulmonary bypass time, risk, and improved outcomes. The hybrid approach to hypoplastic left heart syndrome serves as a role model of the concept (Figure 1) (1, 10).
Hybrid therapy for valve disease
Transcatheter valve therapies are currently developed for the most common valve diseases: mitral valve regurgitation, aortic stenosis, and – in children – pulmonary valve disease. For repair of mitral regurgitation more than 30 devices are currently under investigation and await FDA approval. Experimentally, prostheses for mitral und tricuspid valve replacement are under development and certainly will be available within the next years. Aortic stenosis is the most frequent acquired heart valve lesion currently treated by surgery in developed countries. Conventional aortic valve replacement for aortic stenosis is based upon standardized guidelines with excellent outcomes particularly in younger patients at relatively low-risk and will remain the gold standard for aortic valve replacement in the upcoming years. However, transcatheter techniques have developed to valid alternatives in high risk patients where conventional surgical techniques are considered too invasive and risky (24). Joint recommendations of the European Society of Cardiology and the European Society of Cardiac Surgery consider the hybrid operating room the optimal environment for these new therapeutic options (22).
Coronary artery disease
Routine evaluation of bypass grafts is the first indication for imaging needs in coronary artery bypass grafting. Several groups reported a considerable number of technical bypass graft failures in the range of 13% - 20% that could be diagnosed intraoperatively by angiography and immediately repaired (14, 25). Surgical bypass grafting and percutaneous coronary artery revascularization are traditionally considered isolated options. A simultaneous hybrid approach may allow an opportunity to match the best strategy for a particular anatomic lesion. Revascularization of the left anterior descending artery with the left internal mammary artery has by far the best treatment option in terms of long term results. Integrating this therapy with percutaneous coronary angioplasty (hybrid procedure) offers multi-vessel revascularization through a mini-thoracotomy. Particularly in high risk patients morbidity and mortality decreases in comparison to conventional surgery (7, 16).
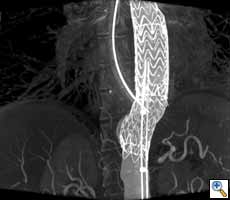 |
| Figure 2. Aortic stent graft visualized with 3D CT-like imaging. Courtesy of Deutsches Herzzentrum Berlin, Germany Courtesy of Prof. Fosse, Rikshospitalet, Oslo, Norway. |
Heart rhythm disturbances
The combination of the surgical epicardial approach with the interventional endocardial approach for the treatment of rhythm disturbances in particular atrial fibrillation offers theoretically advantages over conventional therapy. First reports emphasize the potential benefits (6).
Endovascular aortic repair
Endovascular repair (EVAR) for the abdominal aorta in chronic aneurysms has become a valid alternative to open repair with superior survival (17). EVAR is also increasingly used for the thoracic aorta (Figure 2). In selected cases EVAR in combination with open surgery is even applied for pathologies of the aortic arch and distal ascending aorta (23).
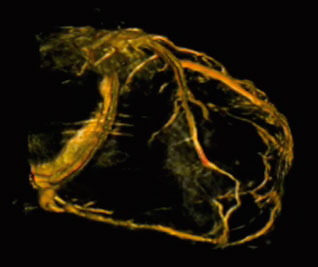 |
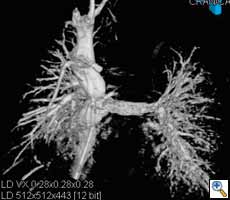
| Figure 3. Visualization of the coronary sinus with 3D CT-like imaging. The coronary sinus can be overlayed over live fluoro and thus offers the surgeons or interventionalists the ability to navigate live in 3D anatomy (3D-Roadmap). Imaging: Courtesy of Dr. Gallagher, Central Baptist Hospital, Lexington, Kentucky, USA |
Pacemaker and ICD implantation
Pacemakers and implantable cardioverter defibrillators (ICD), particularly bi-ventricular systems, may be optimally implanted in a hybrid OR environment, because the hybrid operating theatre offers the required superior angulation and imaging capabilities in comparison to mobile C-arms and the higher hygienic standards compared to cathlabs. (Figure 3)
Interdisciplinary use
The need for hybrid operating theatres is not restricted to cardiovascular disease. Vascular and neuro specialists have equally developed hybrid procedures necessitating angiography systems in a surgical environment. Imaging needs, hygienic requirements, and room set up - particularly for neurointerventions - may be considerable different. Other surgical disciplines may want to introduce navigation systems, magnetic resonance imaging, endoscopy, biplane angiography systems, or a lateral position of anesthesia equipment. However, the hybrid operating rooms are more commonly shared between cardiac surgeons, interventionalists including cardiologists, interventional radiologists, electrophysiologists, neuroradiologists, and pediatric cardiologists. Their specific needs have to be carefully considered and weighted when planning the hybrid theatre.
Basics of the hybrid room
Location
The hybrid room is used by an interdisciplinary team of surgeons, interventional cardiologists, anesthesiologists, and others and it is good practise to involve all stakeholders deeply into planning and keeping such a facility. Ideally, the hybrid OR is located next to interventional suites and operating rooms in order to keep logistics simple. However, if the ORs are separated from the interventional cath labs it is recommend to establish the hybrid OR next to the other operating rooms (8), because all OR equipment and personnel (e.g. heart-lung machine and perfusionists) are immediately ready and anesthesia and intensive care is available.
Room size and preparation
Interventional rooms have excellent imaging capabilities but frequently lack the prerequisites, size, and equipment required for formal operating rooms. Operating rooms meet those required standards, but usually lack high-level imaging capabilities. A hybrid suite should be larger than a standard OR and the basic principle for planning is the larger the better, because not only the imaging equipment needs sufficient space. Staff calculations have shown that in hybrid procedures 8 to 20 people are needed in the team including anesthesiologists, surgeons, nurses, technicians, perfusionists, experts form device companies and so forth (21). Expert opinions recommend for newly built ORs at least 70 m2 (4). Additional space for a control room and a technical room is mandatory adding up with washing and prep rooms to a total of approximately 150 m2 for the whole area. If a fixed C-arm system is considered, an OR size of 45 m2 is the absolute lower limit. Rebuilding in terms of lead shielding (2-3 mm) will be needed. Depending on the system it may be necessary to enforce the ceiling or the floor to hold the weight of the stand (approx. 650 – 1800 kg).
Planning
Planning of the hybrid room is truly an interdisciplinary task. Clinicians and technicians of all involved disciplines should define their requirements and form a responsible planning team. The concrete planning is refined in several steps by specialized architects, vendors of OR equipment, and imaging systems in a close feedback loop with the planning team. Virtual visualization of the room, visits of established hybrid rooms, and information exchange with experienced users help tremendously during the planning process. In the recent literature a couple of case studies are published for planning guidance (9, 12, 15, 18).
Lights, Monitors, and other devices
In general, all members of the team need access to all important information. Therefore, multiple moveable and flexible booms need to be installed in the hybrid operating room. If there are two booms to be installed, a boom on every side of the table serves the team. Collision of the ceiling-mounted displays with operating lights or other ceiling-mounted equipment should be avoided. Large displays are now available capable of showing multiple video inputs in various sizes and decreasing the need for multiple screens. A dedicated ceiling plan with all ceiling- mounted components including air condition should be drawn to ensure the function and usability of all devices.
Conventional surgical lights may collide with the imaging equipment particularly with ceiling- mounted systems. If a laminar air flow is present in combination with a ceiling-mounted system, light pendants need very long arms making them cumbersome to handle. An alternative may be new light concepts with ceiling-integrated multiple theatre lights, as developed at The Interventional Centre at Rikshospitalet in Oslo, and therefore solve the problem of collision with the imaging equipment. A remote control offers the possibility to focus the light where needed (www.lightor.com) (Figure 4).
Hygiene
Hygienic requirements differ from country to country and even among surgical disciplines with the highest standards in orthopaedic surgery. In order to guarantee highest flexibility in room usage, hospitals tend to equip all operating rooms according to the highest standards and that includes a laminar air flow ceiling. Some hospitals even require skirts around the laminar air flow field and this set up may preclude ceiling-mounted systems. In any case, ceiling mounted systems with running parts above the operating field, which are difficult to clean and interfere with the air flow by causing turbulences, are least recommended from a hygienic stand point (8).
Imaging equipment
Mobile and fixed systems
Mobile C-arms have been commonly used in cardiac surgery and they are readily available in every department, e.g. for pacemaker implantation. Mobile C-arms may depict larger stents or catheters well. However, their technical specifications do not meet the recommendations of the cardiology societies (3). The power (2-25 kW vs. 80-100 kW recommendation) and the frame rate (up to 25 f/s 50 Hz or 30 f/s 60 Hz vs. 30-60 f/s 50Hz recommendation) are below the standards (ACC). The cardiology recommendations are to be met, because cardiologic or neuroradiologic interventions are integral part of the hybrid procedure. Thin guidewires (0.2 mm) and stents must be visualized even in obese patients and stenoses of small vessels have to be quantified, which requires adequate power. Mobile C-arms generally have a heat storage capacity of up to 300,000 heat units (HU) (exception: rare water cooled systems). A heat storage capacity of more than 1 million HU is recommended by cardiology societies for cathlabs to avoid overheating and a dangerous shut down during complex procedures which may occur in mobile C-arms. For these reasons expert consensus recommends use of fixed C-arms (8). A semi-mobile system with a fixed generator (80 kW, AXIOM Artis U; Siemens AG, Forchheim, Germany) may accommodate high-power imaging demands even in average sized operating rooms too small to house a fixed C-arm (<45 m2).
Image intensifier and flat panel detector systems
Modern fixed C-arm systems are equipped with a flat panel detector (FD). Contrast resolution is far higher compared to image intensifier (II) systems leading to a higher image quality in detector systems. Additionally in II systems the image is slightly distorted at the edges compared to the centre. As a consequence II systems are not capable of 3D imaging with soft-tissues contrast resolution.
Ceiling and floor mounted systems
Expert consensus recommends floor-mounted systems for hygienic reasons. In fact, some hospitals do not allow running parts immediately above the operative field, because dust may fall down and cause infections. Despite these facts, a high number of hospitals decide to have ceiling mounted systems as these certainly cover the whole body with more flexibility and – most important – without moving the table, which is a sometimes difficult and dangerous undertaking during surgery, because many lines and catheters have to be moved alongside. Some ceiling-mounted systems are capable of 3D imaging from a surgical position, perpendicular to the patient from both the right and left table side. Moving from a parking to a working position during surgery, however, is easier with a flexible floor-mounted system, because the C-arms just turns in from the side without interference with the anaesthesiologist, whereas ceiling-mounted systems can hardly move during hybrid procedures in the park position at the head side without colliding with the anaesthesia equipment.
Mono- and biplane systems
In an overcrowded environment like an hybrid suite, biplane systems add to the complexity and interfere with anaesthesia except for neurosurgery, where anaesthesia is not at the head side. Monoplane systems (Figure 5) are therefore clearly recommended for rooms mainly used for vascular, cardiac, and orthopaedic procedures. There are certainly exceptions. If paediatric cardiologists, electrophysiologists, or neuroradiologists are important stakeholders in room usage, a biplane system may also be considered.
 |
| Figure 5. Example of a room plan with a robotic floor mounted imaging system. A room size of 70 m2 or larger is recommended for hybrid ORs because of the large amount of equipment and people needed. |
Table considerations
The operating table should meet the expectations of both surgeons and interventionalists. This is in fact a special challenge, because the expectations may be mutually exclusive. Surgeons expect a table with a breakable tabletop. For imaging reasons the table has to be radiolucent and should allow coverage of the patient in a wide range. Therefore carbon fibre tabletops are used that are not breakable. Cardiovascular surgeons in general do not have very sophisticated positioning needs and are used to have fully motorized movements of the table and the tabletop. For the positioning of the patients inflatable cushions are sometimes used for positioning if no breakable table is available. Interventionalists require a floating tabletop to allow fast and precise movements during angiography and in some countries floating tabletops are among the technical requirements for performing coronary angiographies or are at least highly recommended by expert consensus (11). Floating tabletops are not available with conventional OR tables. The radiolucent area of the OR table only meet the needs in paediatric cases – a complete coverage of an adult can not be achieved with today’s systems.
As a compromise, tables with a floating tabletop and vertical and lateral tilt are recommended (21). Special rails for mounting special surgical equipment like retractors or camera holders should be available on the table. Placing the operating table in a diagonal position in the hybrid suite may gain space. A crucial element when selecting the imaging system and table is the possibility to have access to the patient from all sides and tilting of the table both head up and down and sidewise. In order to perform 3D imaging with the operating table the C-arm has to be fully integrated with the table, because a fast and precise rotation around the patient lying in the isocentre is necessary. Breakable OR tables are currently not fully integrated and therefore 3D CT-like imaging on these tables is impossible.
Imaging methods and technologies
Fluoroscopy and acquisition are the basic and most important imaging modes and offered by all systems. Since fluoroscopy needs much less radiation dose, brilliant fluoroscopy images are the predominantly used images during the procedure. However, modern angiography systems offer advanced imaging and post-processing capabilities including image fusion with any type of previously acquired 3D volumes (e.g. CT, MR, PET, SPECT images), guidance, or 3D imaging.
CT-like 3D imaging with the angiography system
Surgery very much depends on 3D visualization of the anatomy and therefore 3D CT-like imaging with the angiography system is an important feature, because it enables the surgeon to navigate in real time in 3D volumes. In principle, CT-like imaging of the heart is performed by one or two sweeps of up to 220° of the C-arm around the patient. During the rotation several hundred images are acquired and then reconstructed as a 3D volume. If the acquisition is gated by an ECG 3D volumes over time can be generated to depict the beating heart. Radiation dose is comparable to a conventional multi-slice CT. The OR staff can move out of the OR completely during a CT-like run, because it lasts only approximately 10 sec. Reconstruction is performed within one minute. Accurate information of the cardiac anatomy in the operating room supports planning of complex procedures like redo operations, surgery for complex congenital heart disease, transcatheter valve replacement. Segmentation of anatomical structures and overlay of the 3D volumes over live fluoroscopy (3D roadmap) enables the surgeon to virtually navigate in 3D anatomy (Figure 2). First investigations demonstrate the value of this new technology in transcatheter aortic valve replacement (13) (Figure 6), particularly with anatomical adjustable valve prosthesis as the Ventor Embracer valves. In pulmonary valve replacement the 3D valve anatomy in relation to the coronary artery anatomy is of great importance to avoid obstruction. In EVAR (5) endoleaks can be evaluated in the operating room and corrected, if deemed necessary.
Conclusions
The hybrid operating room facilitates a whole new spectrum of new cardiovascular therapies and will therefore become an essential resource of every cardiovascular centre. The trend towards hybrid techniques is more a revolution than an evolution due to the rapid integration into the surgical techniques. All areas of cardiac therapies – procedures for ischemic, structural, and rhythm heart disease - are deeply affected. Fluoroscopy represents the basic imaging mode. Furthermore. image fusion, 3D/4D imaging, soft tissue visualization, modelling, and navigation allow very advanced surgical applications. The hybrid operating suite itself represents an extremely complex working environment that demands careful planning by all stakeholders. Bundling of clinical, technical, and architectural expertise as well as a realistic view on what is achievable is key for a successful hybrid OR project. Due to wide variations in utilization generic recommendations only are of limited use for these highly individual rooms and certainly cannot replace the diligent work of the project team. However, once the room is successfully established it really transforms surgical techniques and paves the way for revolutionary new minimally-invasive therapies.
References
- Bacha EA, Daves S, Hardin J, Abdulla RI, Anderson J, Kahana M, Koenig P, Mora BN, Gulecyuz M, Starr JP, Alboliras E, Sandhu S, Hijazi ZM. Single-ventricle palliation for high-risk neonates: the emergence of an alternative hybrid stage I strategy. J Thorac Cardiovasc Surg. 131:163-171.e2, 2006.
- Bacha EA, Marshall AC, McElhinney DB, del Nido PJ. Expanding the hybrid concept in congenital heart surgery. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 146-50, 2007.
- Bashore TM, Bates ER, Berger PB, et al.; American College of Cardiology. Task Force on Clinical Expert Consensus Documents. American College of Cardiology/Society for Cardiac Angiography and Interventions Clinical Expert Consensus Document on cardiac catheterization laboratory standards. A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 37:2170-214, 2001.
- Benjamin ME. Building a Modern Endovascular Suite. Endovascular Today 3:71-78, 2008.
- Biasi L, Ali T, Ratnam LA, Morgan R, Loftus I, Thompson M. Intra-operative DynaCT improves technical success of endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2008 Nov 26
- Bisleri G: Combined Surgical And Interventional Electrphysiology Approaches to Atrial Fibrillation: The Hybrid Ablation Procedure, TCT Meeting Washington 12-15.10.2008
- Bonatti J, Schachner T, Bonaros N, Jonetzko P, Ohlinger A, Ruetzler E, Kolbitsch C, Feuchtner G, Laufer G, Pachinger O, Friedrich G. Simultaneous hybrid coronary revascularization using totally endoscopic left internal mammary artery bypass grafting and placement of rapamycin eluting stents in the same interventional session. The COMBINATION pilot study. Cardiology. 110:92-5, 2008.
- Bonatti J, Vassiliades T, Nifong W, Jakob H, Erbel R, Fosse E, Werkkala K, Sutlic Z, Bartel T, Friedrich G, Kiaii B. How to build a cath-lab operating room. Heart Surg Forum. 10:E344-8, 2007.
- Eagleton, MJ, Schaffer, JL. The Vascular Surgery Operating Room Endovascular Today 8:25-30, 2007.
- Gutgesell HP, Lim DS.Hybrid palliation in hypoplastic left heart syndrome. Curr Opin Cardiol. 22:55-9, 2007.
- Hamm CW, Bösenberg H, Brennecke R, Daschner F, Dziekan G, Erbel R, Ewen K, Geffers C, Hausdorf G, Kelm M, Rüden H, Sauer G, Strauer B; German Society of Cardiology-Heart and Cardiovascular Research. [Guidelines for equipping and managing heart catheter rooms (1st revision). Issued by the governing body of the German Society of Cardiology-Heart and Cardiovascular Research. Revised by order of the Committee of Clinical Cardiology] Z Kardiol. 90:367-76, 2001.
- Hirsch R. The hybrid cardiac catheterization laboratory for congenital heart disease: From conception to completion. Catheter Cardiovasc Interv. 71:418-28, 2008.
- Kapadia SR, Role of CT angiography to evaluate patient eligibility and guide transcatheter aortic valve eligibility. TCT Meeting Washington 12-15.10.2008
- Mack MJ. Intraoperative coronary graft assessment. Curr Opin Cardiol 23:568-72, 2008.
- Peeters P, Verbist J, Deloose K, Bosiers M. The Catheterization Lab of the Future. Endovascular today 3: 94-96, 2008.
- Reicher B, Poston RS, Mehra MR, Joshi A, Odonkor P, Kon Z, Reyes PA, Zimrin DA. Simultaneous "hybrid" percutaneous coronary intervention and minimally invasive surgical bypass grafting: feasibility, safety, and clinical outcomes. Am Heart J. 155:661-7, 2008.
- Schermerhorn ML, O'Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 358:464-74, 2008.
- Sikkink CJ, Reijnen MM, Zeebregts CJ. The creation of the optimal dedicated endovascular suite. Eur J Vasc Endovasc Surg. 35:198-204, 2008.
- Sivakumar K, Krishnan P, Pieris R, Francis E. Hybrid approach to surgical correction of tetralogy of Fallot in all patients with functioning Blalock Taussig shunts. Catheter Cardiovasc Interv. 70:256-64, 2007.
- Sternbergh WC 3rd, Tierney WA, Money SR.Importance of access to fixed-imaging fluoroscopy: practice implications for the vascular surgeon. J Endovasc Ther. 11:404-10, 2004.
- ten Cate G, Fosse E, Hol PK, Samset E, Bock RW, McKinsey JF, Pearce BJ, Lothert M. Integrating surgery and radiology in one suite: a multicenter study. J Vasc Surg. 2004 Sep;40(3):494-9.
- Vahanian A, Alfieri OR, Al-Attar N, et al.. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2008 Jul;34(1):1-8, 2008.
- Walsh SR, Tang TY, Sadat U, Naik J, Gaunt ME, Boyle JR, Hayes PD, Varty K. Endovascular stenting versus open surgery for thoracic aortic disease: systematic review and meta-analysis of perioperative results. J Vasc Surg. 47:1094-1098, 2008.
- Walther T, Chu MW, Mohr FW. Transcatheter aortic valve implantation: time to expand? Curr Opin Cardiol. 23:111-6, 2008.
- Zhao DX, Leacche M, Balaguer JM, et al.: Routine intraoperative completion angiography after coronary artery bypass grafting and 1-stop hybrid revascularization results from a fully integrated hybrid catheterization laboratory/operating room. J Am Coll Cardiol. 2009 Jan 20;53(3):232-41.