Introduction
Double aortic arch is the most common of the complete vascular rings, causing tracheo-esophageal compression. This is a case report of a double aortic arch with balanced aortic arches, presenting with extrinsic tracheobronchial obstruction.
Case Presentation
A 5-month-old male child was referred to our institution with stridor since 1 month of age, frequent interrupted feedings, and recurrent respiratory infection, for evaluation and management.
Chest xray showed no perceptible indentation of the tracheal shadow. Echocardiographic evaluation showed normal cardiac anatomy and a right aortic arch. Aberrant course of the left subclavian artery was suspected.
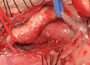
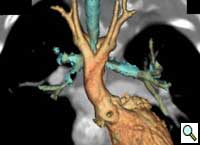
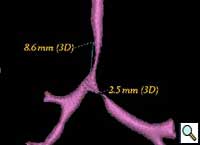
Patient was further evaluated with 64-slice MD CT angiogram which revealed double aortic arch, with balanced arches encircling the trachea and esophagus (Figure 1). The distal trachea and proximal left bronchus were compressed by the vascular ring (Figure 2, 3).
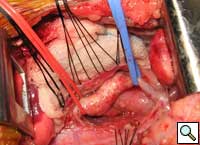
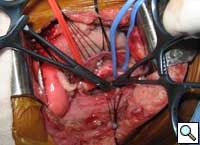
The patient underwent division of the right aortic arch through a left posterolateral thoracotomy (3rd intercostal space) approach. The right aortic arch was posterior, giving rise to the right common carotid and subclavian arteries. The left aortic arch was anterior, giving rise to the left common carotid and left subclavian arteries (Figure 4). The ligamentum arteriosum was attached to the left aortic arch and the left pulmonary artery. The ligamentum arteriosum was divided.
The posterior right aortic arch was clamped just proximal to its junction with the descending aorta. There was no decrease in femoral arterial blood pressure, no gradient between the right radial and the femoral arterial blood pressures, and both radial and carotid pulses were palpable. The posterior right aortic arch was divided at its junction with the descending aorta and the cut edges were oversewn (Figure 5).
 |
 |
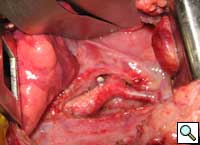 |
| Figure 4. Double aortic arches encircling the esophagus. | Figure 5. Division of the posterior right arch between clamps. | Figure 6. Retraction of the divided aortic arch behind the esophagus. |
The mediastinal tissue around the aortic arches was dissected, allowing the right aortic arch to retract behind the esophagus (Figure 6). Strands of tissue around the trachea and esophagus were divided to relieve any potential residual compression or fibrosis.
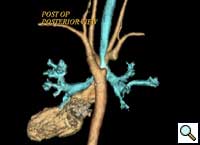
The child was extubated on the first postoperative day. The child was stable and comfortable without any clinical evidence of respiratory obstruction or tracheobronchomalacia. He was transferred to the ward on the second postoperative day. The remaining postoperative recovery was uneventful, with no respiratory or feeding problems. Postoperative CT scan showed widely separated ends of the divided right aortic arch (Figure 7) and relief of airway compression from the vascular ring (Figure 8).
 |
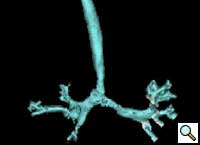 |
| Figure 7. Posterior view: Retraction of the divided ends of the right aortic arch. | Figure 8. Anterior View: Relief of airway compression. |
The patient continues to be free of respiratory compromise and feeding problems at follow-up of one year, and ECHO evaluation revealing a peak gradient of 12 mm Hg at the junction of the left aortic arch with the descending aorta.
Discussion
Complete vascular rings are aortic arch anomalies in which the trachea and esophagus are compressed by the aortic arches and their derivatives. Double aortic arch, first reported in 1737 by Hommel, is due to the presence of right and left aortic arches encircling and compressing the trachea and the esophagus. Robert Gross performed the first successful surgical division of a vascular ring in 1945 at Boston Children’s Hospital through a left antero-lateral chest approach [1]. He further elucidated the surgical principles involved in the division of vascular rings [2].
Embryologically, the ventral and dorsal aortas are connected by aortic arches which persist or involute to give rise to the normal aortic arch, its branches and minor arteries of the head. The right fourth aortic arch normally involutes at about 36 to 38 days in the 16-mm embryo and the left fourth aortic arch persists to give rise to the normal left aortic arch [3]. A schematic depiction with double aortic arches and double ductus arteriosi was described by Edwards to explain the various aortic arch anomalies due to the abnormal persistence or regression of various segments in this hypothetical double aortic arch model [4]. The persistence of both the right and left fourth aortic arches leads to a double aortic arch.
Anatomically, the ascending aorta arises normally and, as it exits the pericardium, it divides into two – right and left aortic arches which encircle the trachea and the esophagus and reunite posteriorly to form the descending aorta. Hypoplasia of one of the aortic arches is common with one arch, more commonly the right aortic arch, being dominant. Atresia can be uncommonly present in any of the segments of either of the aortic arches, resulting in various subtypes of double aortic arch with atresia [5]. The posterior descending aorta formed by the union of the two aortic arches can be on the left or on the right of the thoracic vertebrae. A right dominant double aortic arch has been more commonly observed with left descending aorta and vice versa. The descending aorta has a tendency to be more midline than normal. This abnormal position can cause compression of the airway between the pulmonary artery and the descending aorta postoperatively, leading to persistence of symptoms following surgery [6]. The ductus or ligamentum arteriosum, which is not a part of the vascular ring, runs between the left pulmonary artery, inferior to the junction between the left aortic arch and the descending aorta [7]. The aortic arches give rise to the ipsilateral common carotid and subclavian arteries and the innominate artery is absent.
A contemporary review of the literature regarding double aortic arches is presented in Table 1.
Table 1
| Backer et al (8) |
Alsenaidi et al (9) |
Chunn et al (10) |
Shanmugam et al (11) |
||
|---|---|---|---|---|---|
| No. Of Patients | 113 | 81 | 11 | 29 | |
| Age | 1.4 ± 2.4y | 6 m | 7m* | - | |
| Sex (M:F) | 1.3:1 | 67: 33 | 19:20 | 17:12 | |
| Presenting complaint | |||||
| Respiratory | Most common | 91% | 95%* | Most common | |
| GI | 15% | 40% | 35%* | 8 (27.5%) | |
| Associated cardiac defect | 7% | 17% | 7(24%) | ||
| Surgical approach - Left Thoracotomy | 108 (95.5) | 72 (92%) | 34 (87%)* | 25(86.2%) | |
| Dominant arch | |||||
|
Right |
75% | 71% | 64% | 86.2% | |
|
Left |
18% |
20% |
6.9% | ||
| Balanced | 7% | 9% | 6.9% | ||
| Operative mortality | None | 2 | 2* | None | |
| Postoperative complications | 33%* | ||||
| Respiratory | Most common: Tracheomalacia Bronchomalacia | ||||
| Chylothorax | 9% | 4* | 3(7.6%) | ||
| Follow up period | 1.8y | 12.5m* | 7.1y | ||
| Residual respiratory symptoms | 54% | 47% | 24.1% | ||
*39 congenital aortic arch anomalies including 11 with double aortic arches
Patients with double aortic arch can be asymptomatic or present with symptoms ranging from nonspecific complaints to life threatening respiratory distress. The symptoms of stridorous breathing, dysphagia, a “barky” chronic cough, susceptibility to bronchopneumonia, head retraction, malnutrition, onset during early infancy, and an increase of respiratory distress during feeding were described by Wolman et al [1]. An esophageal foreign body at the site of compression by the vascular ring is rarely the presenting feature of double aortic arch. Life threatening episodes of respiratory arrest and apnea have also been described [9].
Conventional chest x-ray may show indentation of tracheal shadow, retro tracheal opacity, and anterior tracheal bowing. Specific radiological signs have been described for barium esophagography [12]. These include bilateral persistent extrinsic compressions of esophagus in AP view, with the dominant arch causing a deeper and superior indentation and a deep posterior indentation in lateral and oblique views. Barium swallow is diagnostic in the majority of cases. However, MDCT and MRI have become increasingly utilized in the diagnosis and evaluation of aortic arch anomalies, including double aortic arch [8]. The assessment of the arch dominance and surrounding tissues in the mediastinum has improved with these radiological modalities, especially when the barium swallow is negative, there is innominate artery compression, or in complex cases. The four-vessel sign in the superior mediastinum can be seen due to the presence of separate subclavian and common carotid arteries on both sides. Presently angiography is rarely indicated or necessary for adequate evaluation of aortic arch anomalies. Echocardiography is recommended to rule out associated congenital cardiac defects [8, 9, 11, 13].
Repair is achieved through a left posterolateral thoracotomy approach, especially those with a dominant right aortic arch [8-11]. The Mayo Clinic recommends a right posterolateral thoracotomy approach in patients with left aortic arch, right-sided descending thoracic aorta, and right ductus or ligamentum arteriosum; a double arch with atresia of the right posterior segment; or when anastomosis of an aberrant right subclavian artery to the ascending aorta is performed [14]. Backer and Mavroudis [15] recommend that innominate artery compression be approached from the right side, with suspension of the innominate artery to the sternum. Repair through a median sternotomy is recommended when concomitant repair of intracardiac defects is performed [13, 14].
The principles of surgery are essentially the same as described by Gross [3]. The surgical repair includes adequate dissection of the aortic arches and descending aorta, division of the non-dominant aortic arch, division of the ductus or ligamentum arteriosum, and the dissection and division of the mediastinal adventitial bands that may compress the trachea or esophagus.
Post-operative complications include bleeding, vocal cord paralysis, pneumonia, pneumothorax, chylothorax, feeding difficulties, and residual respiratory obstruction. Residual respiratory complaints have been noted in up to 54% of patients [9-11]. While the residual respiratory symptoms are usually due to tracheobronchomalacia, anatomic compression of the trachea by the postoperative arch and the anterior remnant of the divided arch, or by midline descending aorta, can be present [7]. Due to the presence of the dual-sidedness of the balanced aortic arches, these patients have a more midline descending aorta. This results in abnormal stacking of the structures anterior to the spine and leads to extrinsic compression of the left main bronchus between the midline descending aorta posteriorly and the pulmonary artery anteriorly.
In summary, vascular rings, more commonly the double aortic arch, are an important cause of tracheo-esophageal compression, the clinical suspicion and diagnosis of which can lead to early surgical intervention and relief or avoidance of immediate or long term respiratory complications.
References
- Wolman IJ. Syndrome of constricting double aortic arch in infancy: report of a case. J Pediatr 1939;14:527–33.
- Gross RE. Surgical relief for tracheal obstruction from a vascular ring. N Engl J Med 1945;233:586-90.
- Gross RE. Arterial malformations which cause compression of the trachea or esophagus. Circulation 1955;11:124-34.
- Congdon ED. Transformation of the aortic arch system during the development of the human embryo. Contrib Embryol 1922;14:47–110.
- Edwards JE. Anomalies of derivatives of aortic arch system. Med Clin North Am 1948;32:925-49.
- Backer CL, Mavroudis C. Congenital Heart Surgery Nomenclature and Database Project. Vascular rings, tracheal stenosis, and pectus excavatum. Ann Thorac Surg 2000;69(suppl):S308-18.
- Fleck RJ, Pacharn P, Fricke BL, Ziegler MA, Cotton RT, Donnelly LF. Imaging findings in pediatric patients with persistent airway symptoms after surgery for double aortic arch. AJR 2002;178:1275–79.
- Backer CL, Mavroudis C, Rigsby CK, Holinger LD. Trends in vascular ring surgery. J Thorac Cardiovasc Surg 2005;129:1339-47.
- Alsenaidi K, Gurofsky R, Karamlou T, Williams WG, McCrindle BW. Management and outcomes of double aortic arch in 81 patients. Pediatrics 2006;118:e1336-41.
- Chun K, Colombani PM, Dudgeon DL, Haller JA Jr. Diagnosis and management of congenital vascular rings: a 22-year experience. Ann Thorac Surg 1992;53:597–602.
- Shanmugam G, Macarthur K, Pollock J. Surgical repair of double aortic arch: 16-year experience. Asian Cardiovasc Thorac Ann 2005;13:4-10.
- Lowe GM, Donaldson JS, Backer CL. Vascular rings: 10-year review of imaging. RadioGraphics 1991;11:637-46.
- Woods RK, Sharp RJ, Holcomb GW, et al. Vascular anomalies and tracheoesophageal compression: a single institution’s 25-year experience. Ann Thorac Surg 2001;72:434-39.
- van Son JAM, Julsrud PR, Hagler DJ, et al. Surgical treatment of vascular rings: the Mayo Clinic experience. Mayo Clin Proc 1993;68:1056-63.
- Backer CL, Mavroudis C. Surgical approach to vascular rings. In: Karp RB, Laks H, Wechsler AS, eds. Advances in Cardiac Surgery-Volume 9. St. Louis:Mosby- Year Book;1997:29-64.