ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Electrical Isolation of the Pulmonary Veins With Bipolar Radio Frequency Ablation on the Beating Heart
Patient Selection
Currently, only patients with preexisting, paroxysmal atrial fibrillation undergoing OPCAB have been offered an adjuvant off-pump Maze procedure.
Patients with previous thromboembolism or warfarin complication, as well as those with palpitations or antiarrythmic drug intolerance are offered this therapy when undergoing concomitant OPCAB. Patients with long standing atrial fibrillation are currently not candidates for off-pump techniques.
Operative Steps
Perioperative transesphogeal echocardiogram is routinely utilized, specifically to assess for evidence of thrombus in the left atrium or left atrial appendage (LAA), which would mitigate against beating heart ablation. Median sternotomy and conduit harvest are first accomplished and heparin dosage is administered to initiate and maintain an ACT > 350 throughout the procedure. The modified Maze procedure is performed prior to OPCAB.
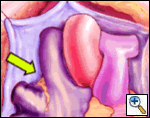 The right side of the pericardium is suspended with stay sutures, and the lateral pericardial attachment to the superior vena cava (SVC) is developed with cautery, allowing better exposure of the right pulmonary artery (RPA) and right superior pulmonary vein (RSPV). The thin pericardial reflection of the inferior vena cava (IVC) is developed, mainly with gentle digital dissection, exposing the posterior lying right inferior pulmonary vein (RIPV).
The right side of the pericardium is suspended with stay sutures, and the lateral pericardial attachment to the superior vena cava (SVC) is developed with cautery, allowing better exposure of the right pulmonary artery (RPA) and right superior pulmonary vein (RSPV). The thin pericardial reflection of the inferior vena cava (IVC) is developed, mainly with gentle digital dissection, exposing the posterior lying right inferior pulmonary vein (RIPV).
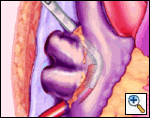 The plane between the RSPV and RPA is developed and a large bronchus clamp inserted between the two and gently manipulated between the IVC and RIPV. This manipulation should require NO force at all. The open end of a 16F Bard Red Robinson catheter is fed into the jaws of the clamp, and the clamp gently withdrawn, effectively isolating the right pulmonary venous drainage. It is important that the open end of the Robinson catheter is cephalad. Development of the interatrial groove is unnecessary.
The plane between the RSPV and RPA is developed and a large bronchus clamp inserted between the two and gently manipulated between the IVC and RIPV. This manipulation should require NO force at all. The open end of a 16F Bard Red Robinson catheter is fed into the jaws of the clamp, and the clamp gently withdrawn, effectively isolating the right pulmonary venous drainage. It is important that the open end of the Robinson catheter is cephalad. Development of the interatrial groove is unnecessary.
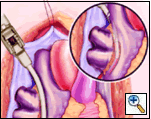 Holding the closed Medtronic bipolar irrigated radiofrequency clamp in the surgeon’s left hand, the malleable clamp tips are gently curved outward. The lower jaw of the open bipolar clamp is placed into the open end of the Red Robinson catheter and the catheter gently withdrawn until the end is visible under the IVC. The catheter is removed and the clamp closed on the atrial side of the pulmonary vein/left atrial interface. Energy is applied until a transmural ablation is assured.
Holding the closed Medtronic bipolar irrigated radiofrequency clamp in the surgeon’s left hand, the malleable clamp tips are gently curved outward. The lower jaw of the open bipolar clamp is placed into the open end of the Red Robinson catheter and the catheter gently withdrawn until the end is visible under the IVC. The catheter is removed and the clamp closed on the atrial side of the pulmonary vein/left atrial interface. Energy is applied until a transmural ablation is assured.
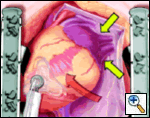 The pericardial sutures are withdrawn and the right hemisternum elevated by placing a folded towel under the lower right side of the sternal retractor, and an apical suction device (Urchin or Starfish [shown at left] Medtronic) attached to the cardiac apex. The heart is gently positioned similar to a ramus or high marginal coronary artery exposure. The left atrial appendage (LAA), and left sided pulmonary system should be in relatively clear view (LPA, LSPV, and LIPV).
The pericardial sutures are withdrawn and the right hemisternum elevated by placing a folded towel under the lower right side of the sternal retractor, and an apical suction device (Urchin or Starfish [shown at left] Medtronic) attached to the cardiac apex. The heart is gently positioned similar to a ramus or high marginal coronary artery exposure. The left atrial appendage (LAA), and left sided pulmonary system should be in relatively clear view (LPA, LSPV, and LIPV).
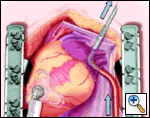 The plane between the LPA and LSPV is developed, and the large bronchus clamp is again passed from cephalad to caudad, with the tip of the clamp being seen caudal to the LIPV. The Robinson catheter is again used to isolate the pulmonary venous drainage on the left side, with the open end of the catheter again being cephalad.
The plane between the LPA and LSPV is developed, and the large bronchus clamp is again passed from cephalad to caudad, with the tip of the clamp being seen caudal to the LIPV. The Robinson catheter is again used to isolate the pulmonary venous drainage on the left side, with the open end of the catheter again being cephalad.
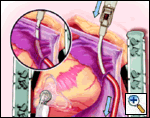 Maintaining the same gentle curve in the clamp, the left-sided pulmonary vein isolation is carried out in a similar fashion to the right side.
Maintaining the same gentle curve in the clamp, the left-sided pulmonary vein isolation is carried out in a similar fashion to the right side.
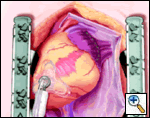 An Ethicon 4.8 mm (Green load) NO KNIFE (NK) endo-GIA stapler is used to remove the LAA, passing the stapler from caduad to cephalad. Ensure hemostasis, though sutures are usually not needed. Total operative time is generally less than 15 minutes. OPCAB is then carried out in the usual fashion.
An Ethicon 4.8 mm (Green load) NO KNIFE (NK) endo-GIA stapler is used to remove the LAA, passing the stapler from caduad to cephalad. Ensure hemostasis, though sutures are usually not needed. Total operative time is generally less than 15 minutes. OPCAB is then carried out in the usual fashion.
Intravenous amiodarone is begun in the OR and converted to oral dosage. Coumadin is restarted once the patient is taking oral medications. Both medicines are continued for at least 3 months and are discontinued only after a Holter monitor shows no episodes of atrial fibrillation.
Temporary pacing wires are placed in the OR and junctional rhythms are promptly atrially paced (though this is usually unnecessary). Recurrences of atrial fibrillation in the postoperative period are expected in a significant number of patients as routine and if persistent, are treated with prompt cardioversion.
The requirement of a permanent pacemaker for sinus node dysfunction is rare with pulmonary vein isolation.
Tips & Pitfalls
-
Patient selection is key as current off pump techniques and lesion patterns are poorly amenable for chronic atrial fibrillation.
-
Preoperative patient discussion about the high likelihood of postoperative atrial fibrillation, the possibility of a pacemaker for sinus node dysfunction (unmasked not created), and the possibility of catheter based techniques for atrial flutter recurrence. It is also helpful to educate your referring physicians about these issues.
-
If TEE shows thrombus in LAA or LA, either abort or convert to an open Maze.
-
Apical cardiac positioners elongate the left ventricle, providing excellent, hands-free hemodynamics and exposure during the left sided ablation.
-
Ensure that saline irrigation is flowing prior to radiofrequency energy application. Foolproof feedback available with the Medtronic bipolar radiofrequency device is invaluable to ensure transmurality.
-
Take great care that all atrial tissue medial to the pulmonary veins is ablated. This may occasionally require a second energy application passing the bipolar clamp from caudad to cephalad to ensure a complete electrical isolation.
-
To avoid potentially torrential bleeding, don’t use the red or blue endo GIA load on the LAA!
-
The “best lesion pattern” for atrial fibrillation is currently unknown and is evolving with the technology. Pulmonary vein isolation may not be sufficient for all patients.
-
Never ablate on the pulmonary veins as pulmonary vein stenosis could ensue. Take great care to ablate only atrial tissue.
References
1. Cox JL, Schuessler RB, Cain ME, et al. Surgery for atrial fibrillation. Semin Thorac Cardiovasc Surg 1989;1:67-73.
2. Benussi S, Pappone C, Nascimbene S, et al. A simple way to treat atrial fibrillation during mitral valve surgery: the epicardial radiofrequency approach. Eur J Cardiothorac Surg 2000;17:524-529.
3. Beating-heart surgical treatment of atrial fibrillation with microwave ablation. Maessen JG, Nijs JF, Smeets JL, Vainer J, Mochtar B. Ann Thorac Surg 2002;74:S1307-S1311.



