Patient Presentation
A 72 year old white male presented with a complaint of recent onset of upper mid-back pain. He states that the pain starts in his chest and radiates to his upper back, and that the symptoms have been relatively constant for the last several weeks. He has intermittently tried taking Tylenol for the back pain without success. He denies any other symptoms such as increased shortness of breath or pain radiating to his left arm. The patient has a history of coronary artery disease, with a remote history of myocardial infarction which was treated with coronary angioplasty and stenting. He has congestive heart failure (CHF) with a recent transthoracic echocardiogram demonstrating an ejection fraction of 20%. In addition, this patient has a history of hypertension and chronic obstructive pulmonary disease (COPD). He has an 80 pack-year history of smoking, but quit smoking approximately 2 years ago.
 |
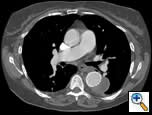
| Figure 1: Contrast-enhanced chest CT scan demonstrating 6-cm aneurysm of the mid-descending thoracic aorta |
 |
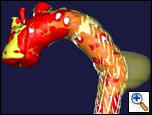
| Figure 2: Three dimensional reconstruction of the chest CT scan |
There were no remarkable findings on physical exam. A chest x-ray revealed a widened mediastinal silhouette, and a high-resolution contrast-enhanced computed tomography (CT) scan was performed using 3mm slices, which demonstrated an isolated 6-cm aneurysm in the mid-portion of the descending thoracic aorta (DTA) (Figure 1). Three dimensional reconstructions were performed from the chest CT scan (Figure 2).
The patient was evaluated for open surgical repair of the aneurysm, but due to his significant co-morbidities, including COPD, CHF, ASA class IV status and severely depressed ejection fraction, he was felt not to be a surgical candidate. Therefore, he was offered endovascular repair of the aneurysm through an Investigator Device Exemption (IDE), Institutional Review Board-approved clinical protocol for the treatment of high-surgical risk patients with DTA pathologies.
After appropriate, informed consent was obtained, the patient was taken to the endovascular suite and underwent induction with general anesthesia. The patient was placed in the supine position on a moveable fluoroscopy table, and the left arm was outstretched and included in the prepped sterile field. An oblique, 5-cm incision was made in the right groin and the common femoral artery was exposed. The right common femoral artery was then directly punctured with an 18-gauge cook needle and, using intra-operative fluoroscopy, a 0.35cm flexible-tipped guide wire was passed in a retrograde fashion into the ascending aorta. A 9-Fr sheath was placed in the right common femoral artery. Five thousand units of heparin were administered intravenously and the activated clotting time was maintained over 200 seconds throughout the remainder of the case. The left common femoral artery was percutaneously accessed and a 5-Fr sheath was placed in the left groin. Similarly, an 0.35cm flexible-tipped guide wire was passed into the ascending aorta in a retrograde fashion.
The patient’s renal function was normal, and therefore a pig-tailed catheter was placed in the aortic arch through the left groin sheath and an aortogram was performed. This demonstrated the aneurysm in the mid-descending thoracic aorta. The proximal neck was adequate and consisted of more than 2 cm of normal aorta proximal to the start of the aneurysm and was sufficiently beyond the origin of the subclavian artery. Likewise, there was an adequate distal landing zone of more than 2 cm of normal aorta beyond the aneurysm. An intravascular ultrasound probe was introduced through the right femoral sheath and the thoracic aorta proximal to the aneurysm measured 31 mm in diameter. Additionally, an arteriogram was performed of the common iliac and external iliac arteries. The arteries were not particularly calcified or tortuous, and therefore appeared suitable to tolerate a large-bore introducer sheath.
We selected a 37 mm diameter by 15 cm in length GORE TAG endoprosthesis to exclude the aneurysm. Therefore, we exchanged the right femoral 0.35 flexible-tipped guide wire for a super-stiff guide wire, and exchanged the 9-Fr sheath in the right groin for a 24-Fr introducer sheath. The device was maneuvered into position and another aortogram was performed to confirm correct graft placement. After lowering the systolic blood pressure to 100 mmHg, the graft was deployed. The distal and proximal ends of the graft were ballooned with a GORE Tri-Flow balloon to gain adequate seal. A completion aortogram was performed which demonstrated adequate exclusion of the aneurysm and no evidence of an endoleak was identified.
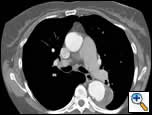
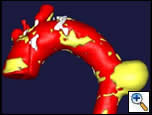
The patient was extubated in the operating room and moved all 4 extremities. He was observed in the Intensive Care Unit for 24 hours, but was able to sit in a chair in the evening of the day of his procedure. The patient commented that his back pain had completely resolved. On post-operative day 2, we obtained a contrast-enhanced high-resolution CT scan to check the position of the graft, and evaluate for endoleaks (Figure 3). The aneurysm was adequately excluded, and there were no evidence of endoleaks. A post-deployment three-dimension reconstruction of the chest CT was performed (Figure 4). The patient recovered uneventfully, and was discharged home on post-operative day 3. Subsequent follow-up has shown no evidence of aneurysmal sac enlargement.
Comments
Despite recent advances in surgical technique with respect to open resection of DTA aneurysms, such as distal aortic perfusion, cerebrospinal fluid drainage and lumbar artery re-implantation, this approach is often associated with significant morbidity associated with the left posterolateral thoracotomy incision [1]. In addition, the risks of neurologic complications associated with traditional surgical techniques are still not insignificant. Many patients with DTA aneurysms also have significant medical co-morbidities that make them high surgical risk candidates and some of these patients are often not able to safely tolerate open resection.
Patients and surgeons are currently seeking safer and less-invasive approaches for the treatment of many surgical disorders. Although endoluminal grafting for abdominal aortic aneurysms has already become an approved treatment option, the first thoracic endoprosthesis (GORE TAG) for the treatment of DTA aneurysms has been approved by the Food and Drug Administration on March 24, 2005.
Initial data from a Phase II multicenter trial using the GORE TAG endoprosthesis suggests that endoluminal grafting for DTA aneurysms is associated with decreased length of stay, less blood transfusion and earlier return to a baseline activity lifestyle [2]. In addition, several studies suggest that endoluminal grafting has an equal, if not less, incidence of neurologic complications [3]. Additional studies are needed to better compare these two different treatment modalities. DTA pathologies such as acute and chronic dissection, penetrating aortic ulcers and aortobronchial fistulas are also potentially amenable to endoluminal grafting [4]. Vascular access is another important consideration when evaluating patients for potential endoluminal grafting. Other issues that need to be examined closer are appropriate management of the sublavian artery when it is covered with an endoluminal graft [5].
As this new technology evolves, associated training issues will become important. Since most practicing cardiothoracic surgeons and residents in training lack the necessary endovascular skills to rapidly adopt this technology, questions arise about how surgeons acquire these skills, and what type and duration of training is necessary. The Society of Thoracic Surgeons has appointed an Endovascular Task Force to deal with these and credentialing issues, and will publish appropriate credentialing guidelines.
References
1. Safi HJ, Miller CC, Huynh TT, et al. Distal aortic perfusion and cerebrospinal fluid drainage for thoracoabdominal and descending thoracic aortic repair: ten years of organ preservation. Ann Surg 2003;238:372-80.
2. Makaroun MS, Dillavou ED, Kee ST, et al. Endovascular treatment of thoracic aortic aneurysms: Results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg 2005;41:1-9.
3. Najibi S, Terramani TT, Weiss VJ, et al. Endoluminal versus open treatment of descending thoracic aortic aneurysms. J Vasc Surg 2002;36:732-7.
4. Thompson CS, Gaxotte VD, Rodriguez-Lopez JA, et al. Endoluminal stent grafting of the thoracic aorta: initial experience with the Gore Excluder. J Vasc Surg 2002;35:1163-70.
5. Tse LW, MacKenzie KS, Montreuil B, Obrand DI, Steinmetz OK. The proximal landing zone in endovascular repair of the thoracic aorta. Ann Vasc Surg 2004;18:178-85.