ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Mitral Valve Repair
Kon N, Riley R. Mitral Valve Repair. October 2022. doi:10.25373/ctsnet.21357597
Patient Selection
Mitral valve repair is preferred whenever technically feasible over valve replacement. Mechanical and biologic prosthetic heart valves have distinct disadvantages. Anticoagulation is required to prevent thromboembolic complications for mechanical valves, and porcine valves have a relatively short life expectancy (7 to 14 years). Long-term data, now past 15 years of follow-up, support the durability of mitral repair.
Operative Steps
Cardiopulmonary Bypass
The patient is positioned and prepped for standard bypass. Exposure is through a full median sternotomy. Arterial inflow is via a single aortic cannula and venous drainage is with bicaval cannulation. Caval snares are used for inflow occlusion during the exposure. Cardioplegia is delivered retrograde into the coronary sinus with a catheter passed transatrially. A left ventricular vent is placed through the right superior pulmonary vein. Care must be taken to avoid dislodging any clot in the left atrium, and therefore we elect to insert the LV vent only after the aortic is cross-clamped. Initially, the vent is left in the atrium or inferior pulmonary veins to aid with a clear visual field. After repair, the vent is gently passed through the mitral valve into the LV. Before weaning from bypass but after confirmation of adequate deairing of the LV, the vent is removed.
Valve exposure
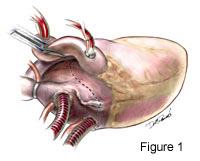
Excellent exposure is keyperforming successful mitral valve exploration and repair. We choose to expose the mitral valve with a superior-septal incision (Figure 1.) This alternative approach accomplishes this well. Not only does the surgeon have uninhibited exposure, but the first assistant also has a clear line of site for the procedure. This is an especially useful when training residents, which obviates the need fortwo surgeons standing on the same side of the table. Exposure is accomplished after inflow caval occlusion andcan be during administration of cardioplegia.The right atriotomy is across the atrial appendage and extends inferiorly parallel to and near the AV groove (see Movie Clip 1 below and Figure 2.) The incision is extended medially and superiorly as well. The left atrium is opened from thefossa ovalis superiorly and vertically across the atrial septum until it joins the right atrial incision. From this point it extends onto the superior dome of the left atrium and underneath the ascending aorta (Figure 3.) 4-0 Prolene traction sutures are placed to aid exposure.
|
![[Figure 2]](/sites/default/files/graphics/experts/Adult/r_riley_MVR/fig2_sm.jpg)
|
![[Figure 3]](/sites/default/files/graphics/experts/Adult/r_riley_MVR/fig3_sm.jpg)
|
TIPS
- Traction is on both sides of the RA appendage and one on the medial side of the transverse portion of the right atriotomy to splay open the RA. These are typically placed before the LA incisions. Two more tractions are placed on the medial side of atrial septal incision and retracted to the patients left. These are also shown in the figure
- Avoid pericardial traction on the left side to allow the heart and pericardium to collapse to the left into the mediastinum.
- Adjust the table with the back and shoulders elevated and the table rotated to the left.
- The assistant may use vein retractors or small Richardson retractors to aid exposure to the mitral valve.
- Care must be taken when making this incision to avoid getting too close to the mitral annulus inferiorly or into the pulmonary veins superiorly. Doing so will potentially make the closure of these incisions difficult.
- See Movie Clip 2.
The SA nodal artery is frequently divided. This approach provides superb exposure and we know of no related permanent complications. Occasional post-operative bradycardia is encountered but resolves spontaneously and we find no increased need for permanent pacemakers.
Mitral valve evaluation
Valve exploration begins with the transesophageal echocardiography (TEE) evaluation. The preoperative TEE not only can be used to determine with a high degree of dependability whether a patient is a candidate for mitral valve reconstruction, but it can also give valuable information with regard to what must be done to fix the valve. The anatomy and mobility of the leaflets, the size of the annulus, that size and direction of regurgitant jets are characteristic for certain valvular pathologies and aid in the planning of the operation. Intraoperative TEE is essential to aid the surgeon with confirmation of a successful repair. Once the valve is exposed, iced saline is injected into the LV and the valve competency and motion assessed. This is again performed after repair to predict success. (See Movie Clip 3) Large valve (blunt nerve) hooks are used to assess the valve leaflets. In addition to assessing the leaflets themselves, the subvalvular anatomy including the papillary muscles and chordae are evaluated. The length of the commissural chords is assessed as well as the relationship between the anterior and posterior leaflets. The mitral annulus must also be assessed for dilatation.
![[Figure 4]](/sites/default/files/graphics/experts/Adult/r_riley_MVR/fig4_sm.jpg) |
![[Figure 5]](/sites/default/files/graphics/experts/Adult/r_riley_MVR/fig5_sm.jpg) |
Annuloplasty
Annuloplasty may be used as sole therapy or in conjunction with other repair maneuvers to support the reconstruction and reinforce the annulus as well as prevent future annular dilatation.
For pure annular dilatation causing mitral regurgitation an annuloplasty reducing the orifice size alone may suffice. This serves to increase leaflet free-edge coaptation. A ring annuloplasty device provides staged plication of the posterior annulus with selective tailoring of more severely involved areas (Figure 4). We prefer a flexible ring such as the Duran® annuloplasty system. The mitral annulus is sized with this system by measuring the distance between the fibrous trigones (Figure 5). Sutures are horizontal mattress with 3-0 Ethibond®. Although a complete ring is depicted in the figure, we often will only perform a posterior annuloplasty and cutout a portion of the ring. Typically the intertrigonal annulus is spared in these circumstances. Sutures are not placed near the AV node or in between the trigone bodies. This technique is mainly used to support other repairs, particularly of the posterior leaflet. It is important, however, to include the fibrous trigones in the annuloplasty.
TIPS
- For pure annular dilatation as the etiology of regurgitation, a complete ring is preferred. This may be sized based on standard body surface areas but generally requires a 27mm to 29mm ring for an adult male and 25mm to 27mm ring for a typical adult female.
- Posterior annuloplasty sutures may be placed early in the valve assessment, which will aid in exposure of the surgical field as well as facilitate placement of subsequent sutures. This maneuver elevates the annulus out of the ventricle and brings the operative field closer to the surgeon.
- The use of suture guides will also allow traction to be placed on these sutures and maintain alignment of the sutures.
![[Figure 6]](/sites/default/files/graphics/experts/Adult/r_riley_MVR/fig6_sm.jpg)
|
Open Commissurotomy
This perhaps may be the best-known technique of mitral reconstruction. With rheumatic valvular disease, mitral stenosis is caused by restricted leaflet mobility. Partial fusion of the commissures with a well-defined border between the anterior and posterior leaflets is ideal (Figure 6). If there is no delineation between the anterior and posterior leaflets or the subvalvular apparatus is fused to the leaflets, there is little long-term success and the valve should be replaced. Of note, in this circumstance, we find that there is little benefit to saving this abnormal subvalvular apparatus during valve replacement. The repair technique requires continued observance of the chordal support mechanism. With traction applied to the major chords of the anterior leaflet near the commisure, a furrow or dimple is created where the leaflets should be incised and separated. This is usually carried out with a No. 15 blade and extends the mitral orifice to within 2mm to 3 mm of the annulus.
![[Figure 7]](/sites/default/files/graphics/experts/Adult/r_riley_MVR/fig7_sm.jpg)
|
![[Figure 8]](/sites/default/files/graphics/experts/Adult/r_riley_MVR/fig8_sm.jpg)
|
Quadrangular resection
Probably the most common situation seen in mitral regurgitation secondary to myxomatous degeneration is prolapse of the middle scallop of the posterior leaflet. This may result from chordal rupture or chordal elongation. Quadrangular resection of the involved middle scallop of the posterior leaflet combined with a posterior mitral annuloplasty is the best way to handle this situation (Figure 7, 8). This quadrangular resection is accomplished by first locating the margins of the involved portion where the chordae are of normal length and structure. A heavy silk tie is passed around these chords to identify and gently retract the section of the posterior leaflet that is not going to be excised. The involved or prolapsed segment is then excised. Advancement flaps are generally then created by cutting along the annulus of remaining posterior leaflet. This creates a sliding plasty of the posterior annulus. The annulus may then be selectively plicated at areas of severe dilatation. Ring annuloplasty sutures are then placed along the posterior annulus. The posterior leaflet is then reconstructed. First, the free edges along the margin of coaptation are identified. A 5-0 polypropylene suture is used to reapproximates these two points. From here, the same suture is run along the body of the leaflet halves back towards the base in a two-layer fashion. The two ends of the suture are then placed through the plicated posterior annulus. (See Movie Clip 4) The same suture, again, is used to attach the leaflet to the posterior annulus in running two-layer stitch.
TIPS
- This leaflet-sliding-plasty technique of creating advancement flaps allows for removal of up to 50% of the posterior leaflet.
- 5-0 Polypropylene works well for the leaflet reconstruction and does not erode into the leaflet
- The suture begins at the free margin of the leaflet and both halves are run towards the annulus.
- To reattach the leaflet, each half of the suture is then run towards one commisure and back to the middle completing a double suture line before any knot is required.
- Placing the posterior ring annuloplasty suture before the leaflet reconstruction elevates the annulus into the wound and improves exposure.
- The annulus may be selectively plicated by focal annuloplasty sutures before the leaflet is reattached.
- The posterior leaflet after mitral repair acts as a doorstop during valve closure for the anterior leaflet to abut against.
- In practice we do not see systolic anterior motion of the mitral valve after posterior annuloplasty.
Triangular Resection
Triangular Resection of the anterior leaflet is may be used for torn chordae tendinae on the anterior leaflet, generally of the central scallop. With a redundant anterior leaflet, this technique may also be helpful. As the name implies, a small wedge or triangle of the anterior leaflet is excised. Our initial experience was to excise a wedge from the free edge of the leaflet back to near the junction with the annulus. However, we now only excise a small triangle of the anterior leaflet and generally do not extend the incision beyond the mid-body of the leaflet. This is closed this primarily with a running 5-0 Prolene suture.
Primary Leaflet Repair
Many of the above mentioned techniques are also useful for repairing a hole in a mitral valve leaflet. If resection of the damaged area necessitates sacrificing major chordae, this becomes unsuitable. The defect in the leaflet may instead be patched with autologous or homologous material. Preshrunk, gluteraldehyde fixed, autologous pericardium may be sewn as a patch covering the hole. Alternatively we have had the occasion to use allograft mitral valve tissue for such a repair. This tissue is not specifically stored or procured for this but may be used in conjunction with allograft aortic valve replacement since the anterior leaflet of the mitral valve usually remains attached with the graft. Occasionally a regurgitant jet of aortic insufficiency secondary to infective endocarditis will create a wind sock deformity in the anterior leaflet of the mitral valve. In this instance, prior to replacing the aortic valve, we have repaired the anterior leaflet of the mitral valve through the aortotomy. Now on the ventricular surface of the anterior leaflet, the excess tissue or vegetation is debrided. The allograft anterior mitral valve leaflet is detached from the aortic root. This is then fashioned to match the size and shape of the native anterior leaflet. A prolene suture is used to attach the allograft tissue to the ventricular surface of the native valve. The suture is run circumferentially around the patch taking care to avoid changing the chordal anatomy and function. Although we are experienced in mitral valve replacement with mitral homograft, we do not perform hemivalve or single leaflet replacement with allograft tissue.
Chordae Tendinae
SHORTENING: We discourage the use of chordal shortening techniques in which a trench is created in the papillary muscle a segment of the elongated chord is buried within the muscle. There are two alternative approaches that we believe are significantly more reliable.
![[Figure 9]](/sites/default/files/graphics/experts/Adult/r_riley_MVR/fig9_sm.jpg)
|
REPLACEMENT: Polytetrafluoroethylene (Gore-Tex) CV-5 can be used to create chordae tendinae in circumstances of elongated or broken chords or when additional chords are required to support the free edge of a leaflet after repair techniques are employed. In particular, when removing a large segment of the posterior leaflet, the remaining chordae form acute angles after sliding annuloplasty (Figures 8 and 9, Movie Clip 5). CV-5 suture is used to create new chords at the central portion of the posterior leaflet. These chords are constructed by passing one of the needles on a double-armed suture twice through the tendinous portion of the papillary muscle that is closest to the free margin of the desired leaflet. Several knots are placed in the suture and then each arm of the suture is passed through the free edge of the leaflet twice. These are placed from the ventricular surface to the atrial side. The sutures are then tied with the knot on the leaflet surface so that the Gore-Tex is the same length as the normal reference chordae.
TIPS
- This is not a recommended procedure for acute mitral regurgitation caused by a ruptured papillary muscle or avulsed chord.
- Gore-Tex requires 9-15 knots to prevent slipping.
![[Figure 10]](/sites/default/files/graphics/experts/Adult/r_riley_MVR/fig10_sm.jpg)
|
![[Figure 11]](/sites/default/files/graphics/experts/Adult/r_riley_MVR/fig11_sm.jpg)
|
TRANSFER: If a medial or paramedial chord is torn or elongated from the anterior leaflet, a corresponding opposing chord from the posterior leaflet is transferred to the anterior leaflet and the defect in the posterior leaflet closed. Chordae of proper length are borrowed from the posterior leaflet and are transposed to the anterior leaflet. The affected chord is excised close to the anterior leaflet contact area. The body of the anterior leaflet is undisturbed. The chosen chords from the posterior leaflet are left attached and a square piece of the leaflet is cut out (Figure 10.) This cutout is then flipped over onto the anterior leaflet so that the two atrial surfaces of the valve leaflets are opposed. A running 5-0 polypropylene suture is then used to approximate these surfaces (Figure 11.) For the posterior leaflet, a focal annuloplasty is performed and the leaflet defect repaired with a running 5-0 polypropylene suture as well.
TIPS
- We do not recommend repairing anterior leaflet prolapse with more than one segment of chordal transfer.
- In essence, a posterior quadrangular resection is performed, without the sliding plasty, as part of this procedure.
Closure
These incisions are closed as follows. A 4-0 prolene suture on an SH needle is run from the apex of the left atrial incision under the aorta, across the dome and carried over the left atrial portion of the atriotomy where the right atrial incisions join, and then continues to close the atrial septum to the fossa ovalis. This is a two layer closure, and the same suture is brought back across the atrial septum and then up the medial portion of the right atrial incision to the appendage. The other arm of the suture is brought out from under the aorta, again across the dome of the LA and now up the medial part of the RA to the appendage where it is tied to the other arm. A second 4-0 is used to close the lateral right atrial incision usually after the cross clamp is removed.
Caval tapes are removed and the heart allowed to reanimate. Again, the vent is usually removed before the heart begins to work (eject).
Conclusion
Mitral valve repair is clearly superior to mitral valve replacement.
Advantages:
- lower operative risk
- better preservation of ventricular function
- lower risk of thromboembolic complications
- less need for anticoagulation
- improved hemodynamic performance
- lower risk for endocarditis
- better long-term survival
- lower costs
Unfortunately, not all valves can be reconstructed. Experience tells us that degenerative valves are most suitable for repair and are associated with the best long-term results. Echocardiography has become an essential tool for establishing the best candidates for repair preoperatively. It aids the surgeon in the intraoperative evaluation of the mitral valve and in the assessment of both the immediate and long-term results of valve repair. These advances in diagnosis, surgical treatment, and follow-up have shown mitral valve repair to be the procedure of choice for many patients with mitral valve disease.
Preference Card
- Medtronic® 20 Fr elongated flexible aortic cannula
- Sarns® 28 FR venous catheter for the SVC
- Sarns® 32 FR venous catheter for the IVC
- 4-0 Prolene® on an SH needle
- 5-0 Prolene® on an RB-1 needle
- Medtronic Duran ® flexible annuloplasty ring
- 3-0 Ticron® suture for the ring
- Gore-Tex® CV-5 for chordae reconstruction
Video
Disclaimer
The information and views presented on CTSNet.org represent the views of the authors and contributors of the material and not of CTSNet. Please review our full disclaimer page here.




