ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
A New Concept For Extensive Use of Neochordae: "The Folding Leaflet"
Three important golden rules for mitral valve repair were postulated by Carpentier and colleagues: preservation or restoration of normal leaflet motion, creation of a large surface of coaptation and stabilization of the entire annulus with a remodelling annuloplasty. Recently, a new paradigm has been proposed by Patrick Perier: “respect rather than resect”. This preservation approach, coupled with the use of artificial chordae, targets correction of prolapse while preserving leaflet tissue to ensure a larger surface of coaptation. But the critical point of neochordal repair is determining the appropriate length of the artificial chordae. Although various maneuvers have been described to ensure safe and reproducible results, accurate chordal height adjustment remains intuitive and based on personal experience. Moreover, leaflet motion is usually impaired when excessive shortening of the neochordae is used to transform the posterior leaflet in a vertical buttress (“frozen” posterior leaflet). We report a simple method for determining the length of artificial chordae preserving both, leaflet tissue and motion, in order to facilitate extensive use of neochordal repair and improve functional results.
This novel concept for mitral valve repair is based on four principles:
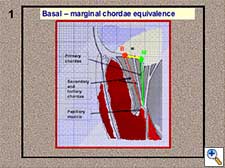
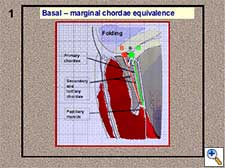
- Basal-marginal chordae equivalence (Figure 1). The height of a marginal (or primary) chordae is equal to that of the corresponding basal (or tertiary) chordae. This is anatomically true in the posterior leaflet, but we can also apply this principle to the anterior leaflet. We can verify this height equivalence by folding the leaflet and moving the marginal chordae to the position of the corresponding basal chordae (Figure 2).
- A pliable leaflet can be folded (Figure 3). The free margin of the leaflet can be temporarily approximated to the adjacent annulus, maintaining both elements in a perfect edge-to-edge position. When the leaflet is completely folded, the annulus is used as the reference point while the neochordae is tied avoiding the sliding of the first knot (Figure 4). In this way, the length of the neochordae will correspond to the height of the basal chordae. After unfolding the leaflet, the neochordae will recover its marginal position and, according to the previous principle, the adjusted basal height will turn into the equivalent marginal height. The length of the neochordae obtained for the prolapsing leaflet will exactly match the plane of the native annulus at the coaptation point.
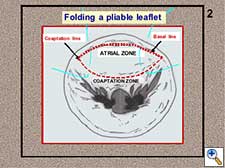 Figure 3. Folding of a pliable leaflet with “folding stitches”.
Figure 3. Folding of a pliable leaflet with “folding stitches”.
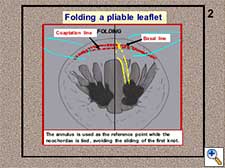 Figure 4. The neochordae is tied during the folding of the leaflet.
Figure 4. The neochordae is tied during the folding of the leaflet.
- Leaflet - papillary muscle segmental correspondence. The insertion of all the native chordae at both papillary muscle groups depicts a C-shaped line and these chordae spread in a fan-like disposition towards both leaflets without crossing the midline (Figure 5). A segmental correspondence exists between the site of native chordal insertion at the level of both papillary muscles and leaflets (Figure 6). Ideally, neochordal repair should reproduce this native anatomical arrangement.
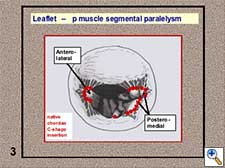 Figure 5. Native chordae C-shaped insertion at both papillary muscle groups.
Figure 5. Native chordae C-shaped insertion at both papillary muscle groups.
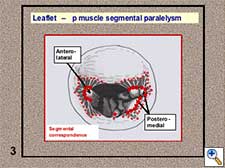 Figure 6. Segmental correspondence between leaflets and papillary muscles.
Figure 6. Segmental correspondence between leaflets and papillary muscles.
- Free edge remodelling by means of a “hockey-stick” effect on the leaflet (Figure 7). The two arms of each neochordae are passed through the free margin of the prolapsing leaflet segment in two or three full-thickness bites on the surface of coaptation. Weaving this suture from the free edge to the atrial surface, surpassing the coaptation line, produces a “hockey-stick” shape to the leaflet, facilitating the creation of a wide surface of coaptation (Figure 8).
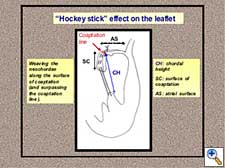 Figure 7. Neochordae implantation: “Hockey-stick” effect on the leaflet.
Figure 7. Neochordae implantation: “Hockey-stick” effect on the leaflet.
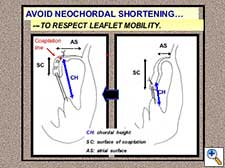 Figure 8. Chordal shortening must be avoided in order to respect leaflet mobility.
Figure 8. Chordal shortening must be avoided in order to respect leaflet mobility.
Several tips on how to use neochordal repair according to this novel concept are presented.
- Implantation of annuloplasty sutures.
- Valve exposure, inspection and segmental analysis.
- Neochordal insertion across the corresponding papillary muscle fibrous tip. The PTFE suture is passed once across this fibrous structure without tying or using pledgets.
- Neochordae insertion on the leaflet along the surface of coaptation, weaving this suture and surpassing the coaptation line to achieve a “hockey stick” effect.
- Folding of the leaflet with the use of folding stitches from the coaptation line to the adjacent annulus (Figure 9). Both, annulus and coaptation line, are maintained in perfect edge-to-edge position.
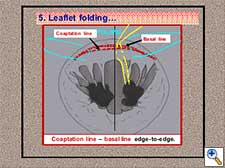 Figure 9. Neochordal height adjustment: leaflet folding.
Figure 9. Neochordal height adjustment: leaflet folding.
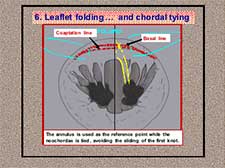 Figure 10. Neochordal height adjustment: chordal tying during leaflet folding.
Figure 10. Neochordal height adjustment: chordal tying during leaflet folding.
- Chordal tying avoiding the sliding of the first knot below the level of the annulus to achieve the correct basal height (Figure 10).
- Unfolding of the leaflet separating the coaptation line from the annulus (Figure 11).
- The neochordae is finally located in its final marginal position with an adequate height resulting in perfect coaptation (Figure 12).
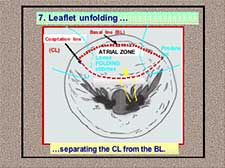 Figure 11. Neochordal height adjustment: leaflet unfolding.
Figure 11. Neochordal height adjustment: leaflet unfolding.
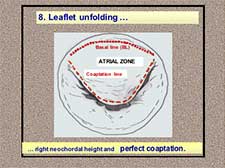 Figure 12. Neochordal height adjustment: leaflet unfolding and final result.
Figure 12. Neochordal height adjustment: leaflet unfolding and final result.
This technique was designed in order to facilitate the extensive use of neochordal repair and improve functional results (Figure 13, 14).
 Figure 13. The main objective: facilitate extensive use of neochordal repair.
Figure 13. The main objective: facilitate extensive use of neochordal repair.
 Figure 14. Complete reconstruction of the mitral valve with multiple neochordae.
Figure 14. Complete reconstruction of the mitral valve with multiple neochordae.
Five surgical videos have been included in this video presentation: three corresponding to mitral reconstructive surgery in fibroelastic deficiency and other two about mitral valve repair in patients with Barlow´s syndrome. We performed the presented neochordal reconstructive technique in all these patients. Complete ring annuloplasty was also used in all of them.
Patient 1. Posterior leaflet prolapse secondary to fibroelastic deficiency.
Male, 52 years old. Asymptomatic. Severe mitral regurgitation. (ERO: 0.52 cm2). LV Ejection Fraction: 58%. Posterior leaflet prolapse: P2-P3 segments with multiple ruptured chordae.
Two pairs of neochordae from the posterior papillary muscle were implanted on the prolapsing posterior leaflet.
Patient 2. Bileaflet prolapse secondary to fibroelastic deficiency.
Male, 76 years old. Symptomatic (NYHA-III dyspnea). Severe mitral regurgitation (ERO: 0.64 cm2). LV Ejection Fraction: 54%. Posterior leaflet prolapse: P2-P3 segments with multiple ruptured chordae. Anterior leaflet prolapse: A3 segment.
Two pairs of neochordae from the posterior papillary muscle were implanted on the prolapsing posterior leaflet. Two additional pairs were also used to correct anterior leaflet prolapse.
Patient 3. Commissural prolapse secondary to fibroelastic deficiency.
Female, 64 years old. Symptomatic (NYHA-II dyspnea). Severe MR (ERO: 0.46 cm2). LV Ejection Fraction: 54%. Posterior commissural prolapse: commissure and A3-P3 segments with multiple ruptured chordae.
Four pairs of neochordae from the posterior papillary muscle group were implanted around the posterior commissure on A3 and P3 leaflet segments.
Patient 4. Posterior leaflet prolapse secondary to Barlow´s syndrome.
Female, 58 years old. Symptomatic (NYHA-II dyspnea). Severe MR (ERO: 0.48 cm2). LV Ejection Fraction: 62%. Posterior leaflet prolapse with excess of tissue: P2-P3 segments with elongated and multiple ruptured chordae.
Four pairs of neochordae from both papillary muscle groups were implanted on P2 and P3 leaflet segments. The height of the posterior leaflet was reduced moving the excess of tissue from the atrial to the coaptation zone during the insertion of the neochordae. In case of excess of tissue, the position of the coaptation line must be moved closer to the annulus in order to decrease the atrial surface of the leaflet (Figure 15). Neochordae are implanted along the surface of coaptation, weaving the Gore-tex suture and surpassing this new coaptation line (Figure 16).
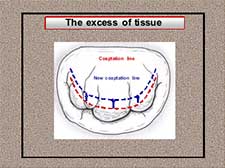 Figure 15. The excess of tissue: moving up the coaptation line closer to the annulus.
Figure 15. The excess of tissue: moving up the coaptation line closer to the annulus.
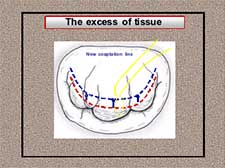 Figure 16. The excess of tissue: weaving the neochordae along the surface of coaptation and surpassing the new coaptation line.
Figure 16. The excess of tissue: weaving the neochordae along the surface of coaptation and surpassing the new coaptation line.
Two objectives must be considered after extensive neochordal repair without resection of tissue in patients with Barlow´s Syndrome:
- The final global atrial surface of the mitral valve must not exceed the internal orifice area of the selected annuloplasty ring (Figure 17).
- The height of the posterior leaflet must be lower than 2 cm (Figure 18).
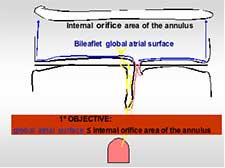 Figure 17. The excess of tissue: the final atrial surface must not exceed the internal orifice area of the annuloplasty ring.
Figure 17. The excess of tissue: the final atrial surface must not exceed the internal orifice area of the annuloplasty ring.
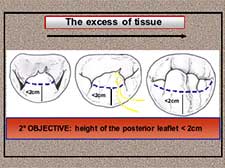 Figure 18. The excess of tissue: the final posterior leaflet height must be lower than 2cm.
Figure 18. The excess of tissue: the final posterior leaflet height must be lower than 2cm.
Patient 5. Bileaflet prolapse secondary to Barlow´s syndrome.
Male, 52 years old. Symptomatic: NYHA-III dyspnea. Severe MR (ERO: 0.52 cm2). LV Ejection Fraction: 58%. Bileaflet prolapse with excess of tissue and elongated chordae.
Several neochordae from both papillary muscle groups were implanted on both leaflets. The excess of tissue was managed according to the presented technique.
Video:




Comments