ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Thoracoscopic Decortication
Komanapalli C, Sukumar M. Thoracoscopic Decortication. December 2011. doi:10.25373/ctsnet.21355452
Index
The ability to completely drain the thoracic cavity, break up loculations of pleural fluid, completely visualize all aspects of the pleural space, and avoid the morbidity of a thoracotomy has made thoracoscopy attractive in the management of empyema and hemothorax.
Patient Selection
The ability to completely drain the thoracic cavity, break up loculations of pleural fluid, completely visualize all aspects of the pleural space, and avoid the morbidity of a thoracotomy has made thoracoscopy attractive in the management of empyema and hemothorax.
Table 1: Laboratory criteria for Drainage of Empyema [1]
| Characteristics of patients that indicate that an invasive procedure will be necessary for its resolution include the following: |
|---|
| An effusion occupying more than 50% of the hemithorax or one that is loculated; |
| A positive Gram stain or culture of the pleural fluid; |
| A purulent pleural fluid that has a pH below 7.20 or a glucose below 60, |
| A lactic acid dehydrogenase level of more than three times the upper normal limit for serum. |
Patients proven to have an infected pleural effusion by thoracentesis and who satisfy laboratory criteria for intervention are candidates for thoracoscopic decortication.
When the empyema is in the exudative or fibrinopurulent stage and has been present for approximately 3 weeks duration or less, thoracoscopic intervention is usually successful (Video 1, Video 2 below). When the empyema has been present for longer than 3 weeks (organizing phase), the ability to perform an adequate decortication may be more difficult due to denser adhesions and the presence of an adherent pulmonary visceral peel [2, 3]. Patients with an exudative or fibrinopurulent empyema can almost always be approached with thoracoscopy; CT scan is not helpful in predicting those patients who will require thoracotomy. Conversion to open thoracotomy is performed when necessary and should not be considered a failure of thoracoscopy, but rather an exercise of mature surgical judgment [4]. Thoracoscopy is also indicated when the nature of the pleural process is undiagnosed, as this allows for a directed pleural biopsy that is likely to make the diagnosis while avoiding the morbidity of a thoracotomy. Other indications for thoracoscopic decortication include hemothorax [5] and mediastinal debridement in patients with descending mediastinitis [6].
Prior thoracotomy, prior talc pleurodesis, and previous empyema are relative contraindication to thoracoscopy. The inability to tolerate single lung ventilation and the presence of a fibrothorax are contraindications to performing thoracoscopic decortication. Patients who develop empyema following esophageal perforation should not be managed thoracoscopically but by thoracotomy.
CT scan of the chest provides information on the location, degree of loculation, the extent of the empyema, and the underlying lung parenchyma (Figure 1). It is not unusual for an organism not to be identified on the pleural fluid culture and therefore broad-spectrum antibiotic coverage should be instituted when the diagnosis of empyema is made. This can be modified if the culture data identifies an organism. The antibiotics are continued for the perioperative period.
An assessment of the patient’s nutritional status should be made and supplemental feedings are initiated if necessary. Bronchoscopy should be performed prior to decortication to rule out endobronchial obstruction in the portion of the lung that is trapped by the empyema.
Operative Steps
General anesthesia is instituted and lung isolation is accomplished using a double-lumen endotracheal tube or a single lumen tube with a bronchial blocker. This allows for expansion and collapse of the lung as needed during the decortication. Intra-operative monitoring includes an arterial pressure line, large bore intravenous access, a Foley catheter, and pulse oximetry. The patient is positioned as for a posterolateral thoracotomy.

Figure 3. Postoperative chest radiograph after VATS decortication, with left pleural drainage tubes in place.
The camera port is placed in the 7th or 6th intercostal space in the line of the anterior superior iliac spine or just anterior to this. VATS decortication and/or evacuation of hemothorax can be performed through 2 or 3 ports. The working port should be placed over the 5th intercostal space between the mid and anterior axillary lines. (Figure 2a) The intercostal incision should allow 3 fingers. A Weitlaner is used to retract the soft tissues (Figure 2b). A third port can be placed posteriorly, positioned to allow access to the anterior part of the pleural cavity.
Once the chest is entered, a Yankauer suction is used to drain the chest of effusion or blood (Video 3, Video 4 below) and along with a finger used to break up simple loculations (Video 5 below). The preoperative CT scan helps guide this ‘blind’ initial drainage and creates a working pleural space for the thoracoscopic instruments. Gelatinous fibrinous deposits and blood clots are removed with a curved ring forceps clips (Video 6, Video 7 below). The visceral pleural peel can be debrided using ring-forceps, a curette (Video 8 below) and a peanut dissector as in an open decortication.
Once a pleural space has been created the removal of fibrinous material is performed over the lateral part of the pleural cavity starting from the apex of the lung and proceeding to the diaphragm or vice versa. The sucker and ring clamp are used together to remove the fibrinous material from the pleural cavity and the curette, peanut and ring clamp are used to dissect the rind on the lung. At the inferior aspect of the pleural cavity it is helpful to identify and separate the lower lobe of the lung from the diaphragm (Video 9 below). This plane is developed posteriorly and anteriorly allowing for the lung to fill the costodiaphragmatic sulcus once the decortication is complete. Next, the posterior aspect of the pleural space is debrided and the underlying lung is decorticated. Exposure is facilitated by rolling the patient anteriorly. Finally, the anterior aspect of the pleural cavity is debrided and the lung freed from where it is adherent to the mediastinum. This exposure is improved by rolling the patient posteriorly. On the left care must be taken to protect the phrenic nerve.
Intermittent ventilation of the lung is used to assess the completeness of the decortication as the dissection proceeds. If adequate progress is not being made or there is inadequate expansion of the lung to fill the chest, then conversion to open decortication should be performed. Particular care should be taken with hemostasis (Video 10, Video 11 below) both on the parietal and visceral pleura.
Once adequate debridement has been accomplished, irrigation is performed and the lung expansion is visualized to ensure the pleural cavity is filled by the lung (Video 12 below). Chest tubes can be placed anteriorly and posteriorly for air and fluid drainage. If there is a small space over the diaphragm a right angle tube is placed in this position to ensure drainage and allow for gradual lung expansion.
The chest tubes are maintained on suction to make sure there is complete lung expansion and adequate drainage of the pleural space (Figure 3). Once the drainage is less than 200cc/24hrs the tubes can be removed. For patients with an empyema, intravenous antibiotics are continued during the postoperative course and for a further 14 days of oral antibiotics once the patient is discharged. For patients with hemothorax, antibiotics are continued postoperatively for 48 hours.
Preference Card
- 30 degree Thoracoscope
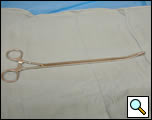
- Curved Thoracoscopic ring clamp (Figures 4a, 4b)
- Yankauer sucker
- Peanut Dissector
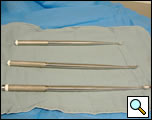
- Long Curettes (Figure 5)
Tips & Pitfalls
- Use a sucker / finger / tonsil sponge stick to “blindly” create a working space in the pleural cavity.
- Check that the lung re-expands to fill the chest adequately before leaving the operating room.
- If uncertain about progression of the operation, bleeding, size of air leak and lung re-expansion proceed to open decortication.
Results
Thoracoscopic decortication is now proven in the management of empyema and the time elapsed since the origin of the empyema and the surgical intervention is probably most predictive of the ability to perform the operation successfully [3, 7]. CT scan is not useful in this respect but helps with port placement and the direction of the decortication within the chest [4]. The principles of open decortication should be followed while utilizing thoracoscopic technology. All areas of fibrinous material must be drained and the underlying lung freed from a restricting pleural peel to allow for complete re expansion. Success rates are high and chest tube duration, hospital length of stay, postoperative pain, and recovery are improved over thoracotomy. Mortality rates are low as are recurrent rates and complications [2-4, 7-9]. Conversions to open thoracotomy are more frequent than after other thoracoscopic procedures, but this should be considered an exercise of sound surgical judgment rather than a failure of the technique.
Thoracoscopic decortication extends the versatility of the thoracic surgeon in his dealing with pleural space infections and can be used for diagnosis and treatment of the same with excellent outcomes.
Video 1
Video 2
Video 3
Video 4
Video 5
Video 6
Video 7
Video 8
Video 9
Video 10
Video 11
Video 12
References
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006;3:75-80.
- Cassina PC et al. Video-assisted thoracoscopy in the treatment of pleural empyema: stage-based management and outcome. J Thorac Cardiovasc Surg 1999;117:234-8.
- Lardinois D et al. Delayed referral and gram-negative organisms increase the conversion thoracotomy rate in patients undergoing video-assisted thoracoscopic surgery for empyema. Ann Thorac Surg 2005;79:1851-6.
- Roberts JR. Minimally invasive surgery in the treatment of empyema: intraoperative decision making. Ann Thorac Surg 2003;76:225-30.
- Navsaria PH, Vogel RJ, Nicol AJ. Thoracoscopic evacuation of retained posttraumatic hemothorax. Ann Thorac Surg 2004;78:282-6.
- Shimizu K, et al. Successful video-assisted mediastinoscopic drainage of descending necrotizing mediastinitis. Ann Thorac Surg 2006;81:2279-81.
- Hope WW, Bolton WD, Stephenson JE. The utility and timing of surgical intervention for parapneumonic empyema in the era of video-assisted thoracoscopy. Am Surg 2005;71:512-4.
- Luh SP, et al. Video-assisted thoracoscopic surgery in the treatment of complicated parapneumonic effusions or empyemas: outcome of 234 patients. Chest 2005;127:1427-32.
- Wurnig PN, et al. Video-assisted thoracic surgery for pleural empyema. Ann Thorac Surg 2006;81:309-13.
Disclaimer
The information and views presented on CTSNet.org represent the views of the authors and contributors of the material and not of CTSNet. Please review our full disclaimer page here.