ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
VATS Lobectomy for Early Stage Lung Cancer
Patient Selection
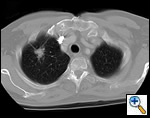 |
| Figure 1: Computed tomography showing a typical right upper lobe lung lesion. surrounded by lung parenchyma suitable for VATS lobectomy |
VATS lobectomies are performed on patients with clinical stage I lung cancer assessed by computed tomography (CT) and positron emission tomography (PET). The procedure is usually performed for tumors less than 4 cm in maximum diameter, but we have resected tumors as large as 6.5 cm. This procedure can be performed regardless of tumor location within the lobe; however, procedures on very proximal hilar tumors are converted to open thoracotomy for better assessment whenever pneumonectomy is contemplated. Current contraindications to performing this procedure include chest wall involvement or surgery planned after neoadjuvant chemotherapy for N2 disease. Prior thoracotomy is not an absolute contraindication since the degree of adhesions and the ability to mobilize the lung adequately will vary among patients. The degree of emphysema, comorbidities, and age are not contraindications, and patients so affected are not managed differently than patients undergoing standard thoracotomy. Figure 1 demonstrates a computed tomography of the typical lesion that is amenable to VATS lobectomy.

Operative Steps
Positioning and port placement
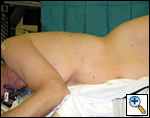 |
| Figure 2: Patient in wrong position because hip would obstruct downward movement of camera |
The patient is placed in the lateral decubitus position with the table break maximally-flexed at the patient’s hip level, and then reverse Trendelenburg tilts the table so the patient’s lateral chest wall is parallel to the floor. Figure 2 shows the patient in a suboptimal position where the hip would limit adequate visualization of the hemithorax by obstructing downward movement of the thoracoscope. Figures 3 and 4 demonstrate the same patient in the proper VATS lobe position. Single lung ventilation is established and the camera port is placed at the eighth interspace in the anterior axillary line for right-sided lesions and in the posterior axillary line for left-sided lesions. The posterior port is then placed where the lower lobe edge touches the diaphragm (approximately the 8th interspace). A lung clamp used as a retractor is placed through the posterior port and the upper lobe is retracted laterally to allow visualization of the superior pulmonary vein (Figure 5). The utility incision (no larger than 4 cm in length) is placed directly over the superior pulmonary vein for upper lobectomies (approximately the 3rd or 4th interspace, and one interspace lower for middle and lower lobectomies. A Weitlaner is used to retract the soft tissues; there is no need for rib spreading (Figure 6).
Right Sided Resections
Right upper lobectomy
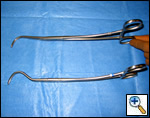 |
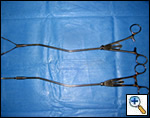
| Figure 7: Harken clamps, long and short |
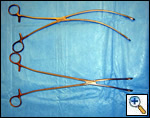 |
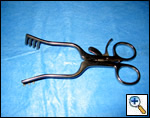
| Figure 8: Curved ringed forceps, long and short |
The superior pulmonary vein is dissected from the overlying pleura via the access incision in the same manner as in an open lobectomy. The perivascular tissue is placed on tension by forceps and standard scissors are used to cut and dissect the tissue in order to expose the superior and inferior aspects of the vein. A curved clamp (Figure 7) is passed behind the superior pulmonary vein after clear identification of the middle lobe vein. The superior pulmonary vein is encircled with a monofilament tie and retracted upwardly via the utility incision. A ring forceps (Figure 8) is placed through the utility incision and is used to retract the upper lobe posteriorly. A vascular (2.5 mm staple height) linear cutting stapling device is placed through the posterior port and, using the monofilament suture as a guide, is passed behind the superior pulmonary vein, and is fired. Once the pulmonary vein has been divided, the truncus arteriosus is easily visualized. Dissection is performed by placing tension on the perivascular tissue with a forceps and incising the tissue with a scissors until the entire arterial branch can be seen from its origin from the main pulmonary artery. The arterial dissection is facilitated if the level 10R lymph nodes are removed first. Once the entire artery is visualized, a curved clamp is passed and a monofilament suture is placed behind the artery and brought through the utility incision. The vascular stapler is passed though the posterior port to transect the vessel. Transection of the truncus artery branch exposes the right upper lobe bronchus. A scissors is used to sharply incise the peribronchial tissue and a blunt pushing motion with closed scissors helps to develop a plane between the interlobar pulmonary artery and the bronchus. Once the interlobar artery is safely pushed away from
 |
| Figure 9: Surgical tissue pouch |
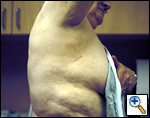 |
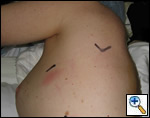
| Figure 10: Postoperative photograph of incisions |
the bronchus and the inferior and superior aspects of the right upper lobe bronchus are clearly visualized, a curved clamp is passed behind the bronchus. A monofilament suture is passed around the bronchus and retracted through the utility incision. A 4.8 mm linear cutting stapler is placed through the posterior port and the right upper lobe bronchus is transected. This exposes the recurrent branch of the pulmonary artery which is transected in the same fashion through the posterior port. Once all the structures to the upper lobe have been divided, the fissure is then assessed. A retractor placed through the posterior port is used to retract the middle and lower lobes inferiorly and a retractor though the utility incision retracts the upper lobe superiorly. Once the fissure is exposed with a long curved ring forceps, a universal 4.8 mm stapler is placed through the utility incision to complete both minor and major fissures. The lobe is then placed in a Cook Urological surgical tissue pouch and removed via the utility incision (Figure 9). Figure 10 shows the incisions postoperatively.
Right lower lobectomy
The lower lobe is retracted superiorly, the pulmonary ligament is transected, and the level 9R lymph nodes are removed. Once the inferior pulmonary vein has been dissected, an endovascular stapler is placed via the utility incision to transect the vessel. The lower lobe bronchus is exposed from its inferior aspect to its bifurcation with the middle lobe bronchus. The bronchus is left intact until the interlobar pulmonary artery is exposed medially and superiorly within the fissure. After the pulmonary artery has been adequately exposed, the bronchus is transected with a 4.8 mm universal stapler placed through the utility incision. The pulmonary artery is then transected followed by division of the fissure via the utility incision.
Right middle lobectomy
The middle lobe is retracted laterally and the pleura overlying the middle lobe vein is incised. Once the vein is dissected it is transected by an endovascular stapler placed via the utility incision, exposing the middle lobe bronchus. After encircling the bronchus with a monofilament suture, an Endo-GIA 3.5 stapler is placed via the utility incision to transect the bronchus. A ring forceps is then placed on the middle lobe bronchus for traction, exposing the one or two branches of the middle lobe artery, which are then transected from the utility incision. On occasion, the angle is such that the middle lobe artery must be transected from the posterior port. The fissures are then completed by passing staplers via the utility incision.
Left-Sided Resections
Left upper lobectomy
The left upper lobe is retracted laterally and the vein is transected from the posterior port. The first branch of the pulmonary artery is dissected and further exposure is obtained by removing the level 10L nodes. The artery is transected via the posterior port. The bifurcation of the left upper and lower lobe bronchi is identified and the left upper lobe bronchus is transected from the posterior port by a universal stapler. A ring forceps is then used to retract the specimen stump of the bronchus laterally, which facilitates exposure of several branches on the pulmonary artery including the lingular artery. These are then transected individually via the posterior port. The fissure is then completed by passing a universal stapler via the posterior port.
Left lower lobectomy
The lower lobe is retracted superiorly, the pulmonary ligament is transected, and level 9L lymph nodes are removed. Once the inferior pulmonary vein has been dissected, a stapler is placed via the utility incision to transect the vessel. The lower lobe bronchus is exposed from its inferior aspect to its bifurcation with the upper lobe bronchus. The bronchus is left intact until the interlobar portion of the pulmonary artery is exposed medially and superiorly from the overlying fissure. After the pulmonary artery has been adequately exposed, the bronchus is transected with a 4.8mm universal stapler placed through the utility incision. The pulmonary artery is then transected via the utility incision and the fissure is completed via the posterior port. Sometimes the superior segmental branch of the pulmonary artery originates from a very proximal location and may require separate dissection and division. In cases with a very thick incomplete fissure, the fissure between the lingula and lower lobe should be completed prior to dissection of the pulmonary artery to facilitate subsequent exposure.
Management of Mediastinal Lymph Nodes
A complete mediastinal nodal dissection or sampling is usually performed whenever a VATS lobectomy is performed for malignancy. For right sided resections, we routinely dissect levels 4R and 7 for upper lobes, and also include level 9R for lower lobes. For left sided resections we routinely dissect levels 5, 6, and 7 for upper lobes and also include level 9L for lower lobes. Our technique of thoracoscopic mediastinal nodal dissection will be described in a future technique section.
Preference Card
- 30 degree rotating scope
- Long curved ring clamp
- Short curved ring clamp
- Harken (curved) clamps, long and short
- Monofilament tie (3-0 PDS)
- Long Metzenbaum scissors
- DeBakey forceps
- Weitlaner retractor
- Sponge stick with open thoracotomy tray (in the event of bleeding the sponge stick is used to tamponade the bleeding while the chest is opened)
- 5”x 8” surgical tissue pouch, Cook Urological Inc, Spencer, IN, USA)
Tips & Pitfalls
- Always have a sponge on a stick, an open thoracotomy tray, and a fine Prolene suture ready for emergency control of hemorrhage
- Prior to bronchial transection, inflate remaining lobes to be sure that the mainstem bronchus has not been mistakenly dissected
- Alternate between panoramic and close up views frequently to prevent too distal or too proximal dissection errors.
Results
We have performed over 130 VATS lobectomies over a two year period at Memorial-Sloan Kettering without any intraoperative surgical or hospital mortality using the techniques described [1]. Oncologic results appear similar to those of standard thoracotomy based on the experience of McKenna et al. in 298 patients [2]. A learning curve exists and significant progress in technique can be expected after approximately 15 procedures. There have been no specific complications as a result of this technique that are not seen after open lobectomy. Pulmonary complications and atrial fibrillation appear to occur with similar frequency as with open lobectomy, although we have not performed a matched comparison to date. Overall, pain appears to be less in the VATS lobectomy patients than in the thoracotomy patients and this potential advantage is currently being studied at our institution.
Acknowledgment: The author would like to thank Robert McKenna, MD for allowing him to observe his operative technique.
References
- Flores RM and Rusch VW. Video-Assisted Thoracic Surgery. In: Wilmore DW, Cheung LY, Harken AH, Holcroft JW, Meakins JL, Soper NJ. ACS Surgery: Principles and Practice. Web MD 2003.
- McKenna RJ, Wolff RK, Brenner M, et al. Is lobectomy by video-assisted thoracic surgery an adequate cancer operation? Ann Thorac Surg 1998;66:1903-1908.