ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Surgical Management of Primary Mediastinal Germ Cell Tumors
Patient Selection
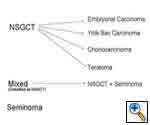
Any anterior mediastinal mass (Figures 1, 2) must be considered suspect for germ cell tumor, especially in a young male. Errors in diagnosis are not uncommon and can result in mismanagement of a potentially curable patient. All patients with an anterior mediastinal mass should have alpha-fetoprotein (AFP), β-human chorionic gonadotropin (β-HCG), and lactate dehydrogenase (LDH) levels drawn at the outset. The different types of germ cell histologies are shown in Figure 3.
A number of hematologic malignancies may occur in conjunction with mediastinal non-seminomatous germ cell tumors (NSGCT), such as acute megakaryocytic leukemia, myelodysplastic syndrome, refractory thrombocytopenia, refractory anemia with excess blasts, malignant histiocytosis, and systemic mastocytosis. In approximately 80% of nonseminomatous germ cell tumors, AFP is elevated. β-HCG is elevated in approximately 30% to 35% of patients and may lead to gynecomastia in a young male patient. Either tumor marker may be elevated alone or together in any particular patient [1, 2]. The presence of any nonseminomatous element (i.e. elevated AFP), even in a tumor that is predominantly seminomatous by histology, is classified as a nonseminomatous germ cell tumor and treated as such. Patients with pure seminomas should never have an elevated serum AFP; its presence implies the presence of yolk sac tumor and embryonal cell carcinoma in the primary or in a metastatic site. In mature teratomas, AFP, B-HCG and LDH are normal.
Although treatment can be initiated based upon positive tumor marker results, histological diagnosis is recommended. There is a pathologic discrepancy of 6% between histology and fine-needle aspiration (FNA), and difficulty may arise in differentiating germ cell tumor from poorly differentiated carcinoma [3]. Core needle biopsy should be performed when possible and, if surgical biopsy is warranted, an anterior mediastinotomy (Chamberlain) is usually the procedure of choice. In diagnostic dilemmas, tissue should be sent for genetic analysis - specifically the i(12p) chromosomal abnormality, which is a consistent finding in germ cell tumors and rarely observed in other tumors. Chemotherapy is the mainstay of initial treatment and surgery should be viewed as an adjuvant to chemotherapy. Bleomycin, etoposide, and cisplatin (BEP) is the current standard [4, 5]. A rapid decline of tumor marker levels with platin-based chemotherapy treatment is associated with improved response rates and overall survival [6]. Chemotherapy is also indicated post-surgical resection when viable tumor is present in the resected specimen.
After initial treatment with chemotherapy, a patient with tumor marker normalization and a persistent mass on computed tomography is the most favorable candidate for surgical resection. Patients demonstrating a residual mass on computed tomography and persistently elevated tumor markers have been treated with salvage chemotherapy in the past in an effort to obtain normal tumor marker levels prior to surgery. This approach, however, has not improved outcome [5], and currently our practice is to recommend surgery after initial chemotherapy (regardless of persistently elevated tumor markers) in an attempt to achieve complete surgical resection. If patients are to undergo salvage chemotherapy preoperatively, this should be performed in a clinical trial setting. In rare circumstances, post-chemotherapy patients will demonstrate normalized tumor marker levels but no residual mass on computed tomography. Surgery is not recommended in these patients. Instead, they should be followed by serial computed tomography scans.
Operative Steps
Surgical assessment should be obtained prior to chemotherapy as well as after treatment. Attention to the patient’s functional status, hematologic function, and pulmonary function testing [especially the diffusion capacity (DLCO)] is critical. Post-chemotherapy DLCO measurement is essential because of the potential of bleomycin toxicity leading to progressive pulmonary fibrosis. If major anatomic lung resection is anticipated (based upon the pretreatment computed tomography), then the amount of bleomycin administered should be limited.
The procedure is performed with a double lumen endotracheal tube in place. Lung collapse is frequently required in order to facilitate exposure and/or lung resection. FiO2 should be kept to a minimum because of the potential harmful effects after neoadjuvant treatment with bleomycin. If there is any possibility of resection of the superior vena cava (SVC), femoral venous access should be obtained.
Incisions
Median Sternotomy
This is the most common approach for small, centrally located tumors in the anterior mediastinum. The patient is placed in the supine position with both arms tucked to the sides, which allows exposure to the right or left hemithoraces, the lung hila, and mediastinal vascular structures. However, exposure to the posterior aspects of the lung is suboptimal and visualization of the left lower lobe is limited.
Hemiclamshell Thoracotomy
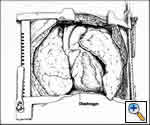
This is our preferred approach for large tumors arising in the anterior mediastinum and extending significantly into either the right or left hemithorax. The exposure is a combined upper median sternotomy and anterior thoracotomy (Figures 4, 5). The patient is placed in the supine position, the side of the anterior thoracotomy extension is elevated 30 degrees with a longitudinal roll placed beneath the scapula, and arms are tucked at the sides. Exposure is facilitated by collapse of the ipsilateral lung, allowing for anatomical lung resection if necessary. Once the involved lung is divided, optimal exposure to the posterolateral aspect of the tumor is obtained; thereby allowing complete assessment of adjacent vascular structures and phrenic nerve.
Hemiclamshell Thoracotomy with neck extension
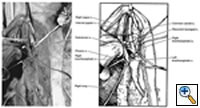
If a large tumor extends into the neck area, an extension along the anterior border of the sternocleidomastoid provides excellent exposure, especially if dissection of the vascular structures is required. This provides excellent exposure of the carotid and jugular vessels (Figures 6, 7).
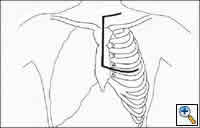 |
| Figure 8: Incision for left hemi-clamshell with supraclavicular extension |
Hemiclamshell Thoracotomy with transverse supraclavicular extension (trap door)
On rare occasions, resection of the subclavian vessels is necessary with mediastinal germ cell tumors. If required, extending the top of the sternotomy along the superior portion of the clavicle allows adequate control of the vessels. In addition, the excision of the medial 1/3 of the clavicle may provide added exposure (Figure 8 ).
Clamshell
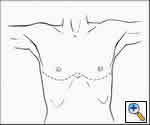
Very large tumors with extension into both hemithoraces are best approached via this method. The patient is placed in the supine position and the arms are either extended or flexed up over the patient’s forehead and secured to the ether screen. A curvilinear incision is made along the inframammary crease, extending from right to left anterior axillary lines (Figure 9). The 4th interspace with transverse division of the sternum usually provides the best exposure; the mammary vessels are ligated and two Finochietto retractors are used to provide retraction (Figures 10, 11). The pleural reflections are incised to gain exposure to the mediastinal structures. If one initially begins with a clamshell incision but finds poor exposure to the superior mediastinum, an upper sternal split may provide added exposure of involved structures.
Posterolateral Thoracotomy
A posterolateral thoracotomy may be used in a select number of cases. However, access to the mediastinal vascular structures is limited and, if resection of these structures is required, conversion to a median sternotomy after dissection of the posterolateral components of the mass may be performed.
Intraoperative decision making: To resect or not to resect?
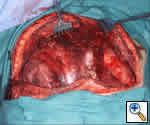
Neoadjuvant chemotherapy is very effective in treating mediastinal germ cell tumors. However, the residual mass becomes intimate with surrounding structures and frequently there are no discernible tissue planes. In these cases, the surgeon is faced with the decision whether or not to resect major structures, with the attendant morbidity versus potentially leaving residual tumor in the chest. Although tissue planes are distorted, one may be able to peel the mass off from structures such as the SVC and phrenic nerve. Some surgeons have recommended shaving a piece of tissue from multiple areas for frozen section analysis. If only fibrosis is evident, then peeling the mass off adjacent structures is reasonable; if it shows tumor, then en-bloc resection is performed. However, many lesions are mixed with necrotic and viable components and may contain seminomatous and non-seminomatous germ cell tumor, teratoma, carcinomatous and sarcomatous elements. Because of this, biopsy of certain areas may not be representative of the entire specimen. Since this method of biopsy is not always accurate, if invasion is suspected intraoperatively, then we recommend resection of technically resectable structures.
Our data showed residual viable tumor in 66% of cases [7]. Therefore, we have a low threshold for resecting phrenic nerve, lung, SVC, pericardium, etc., en-bloc. Dissection is usually started from left to right since the structures on the left are usually considered unresectable (e.g. aorta) and the structures on the right are considered resectable (e.g. SVC). This will allow an assessment as to whether complete resection is possible. If so, a more radical procedure is reasonable. Should the complete removal of all disease not be possible, then the resection of major structures such as the SVC or phrenic nerve may be futile and not worth the attendant morbidity.
When tumor markers have completely normalized, fibrosis will usually be encountered at the periphery. Conversely, when tumor markers persist, it is likely that viable tumor is present. However, there is no steadfast rule in making the decision to resect or not to resect a major structure.
Resectable structures
Thymus
The majority of primary mediastinal germ cell tumors arise in the thymic tissue of the anterior mediastinum. Therefore, resection of the entire thymus gland affords the best chance of complete surgical resection. Because the thymus is a technically easily resectable structure with little consequence to the patient after removal, in the majority of cases we recommend en bloc resection of the thymus along with the germ cell tumor mass.
Pericardium
The pericardium usually comprises the most posterior extent of the germ cell tumor mass. Resection of this structure is not always required for a complete resection; however, in many situations it is resected easily with little downside to the patient. When the involvement of the posterior margin is of concern, the pericardium should be resected en-bloc. In certain cases, the pericardium is reconstructed with Gore-Tex to protect the underlying structures in the event repeat sternotomy is required.
Lung
The lung frequently requires resection in order to obtain negative margins and to gain adequate exposure to the mediastinal structures posterior to the mass. Since only peripheral portions of the lung are in direct contact with the structures of the anterior mediastinum, a wedge resection is usually sufficient in order to obtain negative margins. Non-anatomical resection is advocated rather than lobectomy. In rare circumstances, lobectomy and even pneumonectomy may be required for lesions invading hilar structures. However, every effort should be made to avoid large pulmonary resections when possible.
Very large tumors can compress the lung to the size of a fist and may deceivingly appear to require a pneumonectomy. Once the mediastinal portion is completely mobilized, positive pressure ventilation is applied to the affected lung with the tumor lifted up and off the mainstem bronchus. This maneuver helps to identify the plane of dissection between lung and tumor.
Phrenic nerve
Every attempt to preserve the phrenic nerve should be made. Preoperative pulmonary function tests may help with the intraoperative decision to resect the phrenic nerve. If all tumor can be resected cleanly and only one phrenic nerve prevents complete resection, then the nerve should be sacrificed if the patient's pulmonary function is adequate. When the phrenic nerve is resected, diaphragmatic plication should be performed to prevent lower lobe atelectasis.
Innominate Veins
Transection of the right or left innominate vein alone may be inconsequential or may result in transient arm swelling. If this is the only structure precluding complete resection, then it should be resected en-bloc with the specimen. However, resection of both structures usually warrants graft interposition.
Superior Vena Cava
Tumor can generally be adequately excised from the SVC by sharp dissection. However, if there is obvious invasion of the SVC or any suspicion on preoperative CT scan, then large bore access inferior to the heart (i.e. femoral vein) should be obtained.
SVC resection may be performed as demonstrated by Venuta et al., in a previous thoracic expert technique section [8]. In situations where the SVC is completely resected, the right phrenic nerve usually requires resection as well.
Postoperative Management
If the final pathological evaluation reveals residual viable tumor, postoperative treatment with chemotherapy is recommended.
Post-pericardiotomy syndrome may occur after resection. Symptoms and clinical findings include pain at the tip of the scapula, fever, pericardial effusion, and diffuse ST elevations on EKG. Treatment with Ibuprofen or NSAIDS generally results in improvement.
Preference Card
- Favalaro mammary retractor
- Gore-Tex 1 mm patch for pericardial reconstruction
- Standard thoracotomy instruments
Tips & Pitfalls
- If the phrenic nerve is resected, diaphragmatic plication should be considered.
- Histologic misdiagnosis at presentation is possible. One must be aware of a number of non-germ cell malignancies that may occur within the tumor mass such as rhabdomyo-sarcoma, synovial cell sarcoma, primitive neuroectodermal tumor (PNET), nephroblastoma, and adenocarcinoma.
- Patients with choriocarcinoma as the predominant histology may have tumors with a hemorrhagic tendency. Thus, care must be taken in performing open surgical biopsy, mediastinoscopy, or bronchoscopy because significant bleeding may occur.
- Major surgical exploration for biopsy, subtotal resection, or complete resection should be discouraged because prolonged recovery delays chemotherapy treatment.
- Resection of bilateral phrenic nerves should not be performed.
- SVC resection without should be performed with venous access both above and
below (femoral vein) the heart.
Results
We recently published our series from Memorial Sloan-Kettering of 32 patients who underwent post-chemotherapy surgical resection of mediastinal germ cell tumors [7]. Histologic analysis revealed viable tumor in 66%, teratoma in 22%, and necrosis in 12% of the specimens. Viable tumor included embryonal carcinoma, choriocarcinoma, yolk sac carcinoma, seminoma, and teratoma with malignant transformation to non-germ cell histology (e.g. sarcoma). Since teratoma and residual tumor were found in the majority of resected patients, we maintain an aggressive approach to surgical resection of potentially involved adjacent structures. The Kaplan Meier curve showing the survival of the 32 patients who underwent post-chemotherapy surgery is shown in Figure 12.
References
- Kay PH, Wells FC, Goldstraw P. A multidisciplinary approach to primary nonseminomatous germ cell tumors of the mediastinum. Ann Thorac Surg 1987;44:578-582.
- Nichols CR, Saxman S, Williams SD, et al. Primary mediastinal nonseminomatous germ cell tumors. A modern single institution experience. Cancer 1990;65:1641-46.
- Singh HK, Silverman JF, Powers CN, Geisinger KR, Frable WJ. Diagnostic pitfalls in fine-needle aspiration biopsy of the mediastinum. Diag Cytopathol 1997;17:121-126.
- International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol 1997;15:594-603.
- Loehrer PJ Sr., Gonin R, Nichols CR, Weathers T, Einhorn LH. Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol 1998;16:2500-2504.
- Mazumdar M, Bajorin DF, Bacik J, Higgins G, Motzer RJ, Bosl GJ. Predicting outcome to chemotherapy in patients with germ cell tumors: the value of the rate of decline of human chorionic gonadotrophin and alpha-fetoprotein during therapy. J Clin Oncol 2001;19:2534-2541.
- Vuky J, Bains M, Bacik J, Higgins G, Motzer RJ, Bosl GJ. Role of postchemotherapy adjunctive surgery in the management of patients with nonseminoma arising from the mediastinum. J Clin Oncol 2001;19:682-688.
- Venuta F, Rendina EA, Coloni GF. Surgery of the superior vena cava: resection and reconstruction. General Thoracic Experts' Techniques, CTSNet. Published 12/01/03 http://www.ctsnet.org/doc/8320
- Bains MS, Ginsberg RJ, Jones WG 2nd, et al. The clamshell incision: an improved approach to bilateral pulmonary and mediastinal tumor. Ann Thorac Surg 1994;58:30-33.
- Korst RJ, Burt ME. Cervicothoracic tumors: results of resection by the "hemi-clamshell" approach. J Thorac Cardiovasc Surg 1998;115:286-295.






Comments