ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Thoracoscopic LVRS
Patient Selection
 |
| Figure 1 |
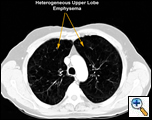
This procedure is performed in patients with emphysema to improve their exercise tolerance and quality of life. Specific criteria were established by the National Emphysema Treatment Trial (NETT) published in 2003. An FEV1 greater than 20% predicted, DLCO greater than 20% predicted, predominantly upper lobe heterogenous distribution of emphysema on CT scan (Figure 1), matching perfusion defect on V/Q scan, low exercise tolerance or less than 40 watts exercise capacity on bicycle exercise testing, and a normal CO2 are all criteria that should be satisfied in selecting patients who will benefit from the procedure [1]. Cooper et al. have established their own set of selection criteria (Table 1) [2].
|
Inclusion Criteria |
Exclusion Criteria |
| General | |
| Disability despite maximal rehabilitation | Inability to participate in rehabilitation |
| Cessation of tobacco use > 6 months | Continued use of tobacco |
| Patient expectation of goals reasonable | Significant co-morbidity |
| Previous pleurodesis or thoracotomy | |
| Underweight, overweight | |
| Anatomic radiographic evaluation | |
| Marked Emphysema | Bronchiectasis |
| Minimal radiographically evident emphysema | |
| Heterogeneously distributed emphysema | Homogeneously distributed emphysema |
| Target zones of poorly perfused lung Areas with better preserved lung |
No target zones No preserved lung tissue |
| Marked thoracic hyperinflation | Chest wall or thoracic cage abnormalities |
| Physiologic Evaluation | |
| Marked airflow obstruction | Minimal to moderate airflow obstruction |
| Marked hyperinflation | Minimal to moderate thoracic hyperinflation |
| Alveolar gas exchange DLCO < 50% | Marked disordered alveolar gas exchange DLCO < 10% PaCO2 > 60 mmHg |
| Cardiovascular Function – essentially normal |
Cardiovascular dysfunction Left ventricular ejection fraction < 40% Mean pulmonary artery pressure > 35 mmHg Significant coronary artery disease |
Patients must go through a pulmonary rehabilitation program involving aerobic exercise, usually on a stationary cycle, and exercises with weights to strengthen the muscles of the chest and upper extremities. Patients are educated in incentive spirometry and sputum clearance prior to surgery. If the patient is on steroids, these must be tapered to the lowest possible dose or discontinued prior to surgery [2].
Operative Steps
 |
| Figure 2 |
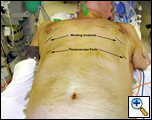
General anesthesia with one lung ventilation is used and both sides are operated on sequentially under the same anesthetic [3]. A working thoracic epidural is mandatory to ensure adequate analgesia so that postoperative pulmonary toilet and rehabilitation can proceed adequately. The patient is placed supine on a bean bag rolled under the back and the arms are partially abducted or tucked by the side (Figure 2). This allows access to both sides of the chest without changing position. Alternately, the patient can be placed in posterolateral thoracotomy position and the position changed sequentially to operate on both sides.
When the patient is supine the surgeon stands opposite to the side being operated on and the table is rolled towards the surgeon. Either side can be operated on first. The bed is flexed and placed in reverse trendelenberg position to open the intercostal space. This improves the access to the thoracic cavity and the upper lobe. The thoracoscope is introduced in the 7th interspace just anterior to the anterior superior iliac spine (ASIS). On the left the scope is introduced into the chest posterior to the ASIS, to avoid injury to the heart and to prevent it from obscuring the view of the operative field.
The working incision is usually placed in the 5th intercostal space just lateral to the midclavicular line (Figure 2). However, the exact position of the working incision can made after visualizing the intrathoracic anatomy once the thoracoscope has been placed. The working incision is usually 5cm in length and the intercostal incision is made a few cm longer. The soft tissues of the incision are spread open using a Weitlaner retractor. No rib spreading is used. This allows the placement of two or more instruments through this incision and allows free entry of air into the chest, keeping the lung collapsed even when suction is applied within the chest (Video 1). Alternatively, the anterior 5th ICS incision can be kept to a 2cm length and a 3rd incision can be made posteriorly in the same space for another instrument.
 |
| Figure 3 |
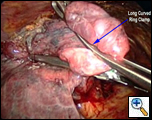
Once the working incision has been made and the lung collapsed, further pressure on the upper lobe parenchyma with a tonsil sponge on a long curved ring clamp ensures complete collapse of the upper lobe. The mediastinal pleura around the upper pulmonary hilum is mobilized sharply or with cautery (Video 2). This allows for increased mobility of the upper lobe. A long curved ring clamp (Figure 3) introduced through the working incision is used to grasp the upper lobe at its apex and retract the lobe superiorly and laterally. This allows the surgeon to inspect the upper lobe and decide the position of the line of resection.
Approximately seventy percent of the upper lobe should be removed. This is achieved by performing a stapled excision of the target area. An Endo GIA stapler with a 60mm 4.8mm load with buttresses is used to create the staple line. The stapler is introduced into the chest through the working incision under the ring clamp and the upper lobe is maneuvered into the jaws of the stapler starting at the anterior edge of the lung. Multiple firings are used to resect the portion of the upper lobe that contains the most diseased areas. As the resection proceeds, the part of the lung being resected is progressively held further posteriorly (Video 3). This ensures that the stapler can be placed accurately to continue the resection line and that an adequate amount of the upper lobe is removed (Video 4). The resected lung is removed through the working incision. If it is too bulky to be easily delivered, it can be placed in a 15mm endobag within the chest and then delivered.
A pleural tent is constructed to drape over the staple line as this helps in sealing air leaks that inevitably develop. The parietal pleura in line with the working incision is incised with cautery down to the level of the intercostal muscle, starting posteriorly just lateral to the descending aorta on the left and the spine on the right, working anteriorly till the incision is encountered. The superior cut edge of the parietal pleura is then grasped gently with a ring clamp posteriorly and mobilized using sharp dissection or bluntly with a peanut dissector (Video 5). The edge of the pleura is further retracted and the pleura is stripped from the chest wall using a tonsil sponge as a blunt dissector (Video 6). This must be done gently and carefully to avoid tearing of the pleura, which makes subsequent dissection difficult and diminishes the quality of the tent. Frequent changing of the tonsil sponge dissector as soon as it becomes soaked with blood is helpful in maintaining its efficacy as a dissector. The dissection is carried anteriorly, posteriorly and superiorly to the apex of the thoracic cavity (Video 7). It is easiest to begin the dissection posteriorly and then work anteriorly.
 |
| Figure 4 |
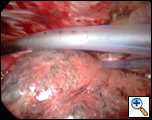
Once completed the pleural tent should hang down to the level of the staple line ensuring that with expansion of the lung it will be covered (Video 8). Hemostasis is secured over the raw surface of the chest wall using cautery, and two 28Fr chest tubes are placed anteriorly and posteriorly (Figure 4). The posterior tube is placed through the thoracoscope incision and the anterior tube through a separate incision placed anterior to this on the chest wall. The lung is then inflated under thoracoscopic vision, with the scope being placed through the working incision to ensure complete expansion of all the lung parenchyma. The opposite side is then operated on in the same way (Video 9).
Preference Card
- 30 degree thoracoscope
- Curved ring clamp
- EndoGIA stapler with 60mm 4.8mm loads with buttress
- Peanut dissector and tonsil sponge
Tips & Pitfalls
- Apply patient selection criteria strictly.
- When operating with the patient supine, the surgeon should stand opposite the side being operated on.
- Mobilization of the mediastinal hilar pleura allows for an easier resection and helps to ensure 70% of the upper lobe is resected.
- Use the 60mm 4.8mm stapler with buttress to resect the lung parenchyma. This also reinforces the staple line, decreasing the air leak.
- Mobilize the parietal pleura gently while constructing the pleural tent using a peanut or tonsil sponge; changing them frequently so they remain relatively dry facilitates the dissection. When adhesions are encountered, this diminishes the quality of the pleural tent or makes it impossible to construct (Video 10).
- Ensure meticulous hemostasis over the area where the parietal pleura has been stripped
- Always place 2 chest tubes to drain the pleural space, and leave the pleurovac on water seal.
Results
The value of LVRS in the treatment of carefully selected emphysema patients is well established; outcomes include improved PFT’s, exercise tolerance, and quality of life for approximately five years [1]. Until recently it was unclear if median sternotomy and a thoracoscopic approach were equivalent. Previous reports have suggested that the thoracoscopic approach greatly decreased the mortality and complication rates compared to median sternotomy [4]. The NETT has demonstrated that both
 |
| Figure 5 |
techniques have a similar outcome in terms of improving quality of life and exercise tolerance at twelve and twenty four months. The thoracoscopic technique allows for a shorter, less expensive hospital stay and a quicker return to daily activities. Ninety day mortality was 5.9% for median sternotomy and 4.6% for thoracoscopic LVRS. The complication rate and morbidities were low for both procedures and did not differ [5]. Thoracoscopic plication or lung compression techniques have not been shown to provide improved results over standard thoracoscopic LVRS. The future of LVRS appears to be in more minimally invasive techniques such as bronchoscopically introduced bronchial valves, bronchopulmonary fenestration, and injection of polymers into the emphysematous portions of the lung to cause atelectasis [6]. However, at this time thoracoscopic LVRS remains a dependable and relatively minimally invasive technique for improving quality of life in select patients with emphysema (Figure 5).
Video 1
Video 2
Video 3
Video 4
Video 5
Video 6
Video 7
Video 8
Video 9
Video 10
References
- Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G and Wood DE: A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73.
- Ciccone AM, Meyers BF, Guthrie TJ, Davis GE, Yusen RD, Lefrak SS, Patterson GA and Cooper JD: Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg 2003;125:513-25.
- Serna DL, Brenner M, Osann KE, McKenna RJ, Jr., Chen JC, Fischel RJ, Jones BU, Gelb AF and Wilson AF: Survival after unilateral versus bilateral lung volume reduction surgery for emphysema. J Thorac Cardiovasc Surg 1999;118:1101-9.
- Roberts JR, Bavaria JE, Wahl P, Wurster A, Friedberg JS and Kaiser LR: Comparison of open and thoracoscopic bilateral volume reduction surgery: complications analysis. Ann Thorac Surg 1998;66:1759-65.
- McKenna RJ, Jr., Benditt JO, DeCamp M, Deschamps C, Kaiser L, Lee SM, Mohsenifar Z, Piantadosi S, Ramsey S, Reilly J and Utz J: Safety and efficacy of median sternotomy versus video-assisted thoracic surgery for lung volume reduction surgery. J Thorac Cardiovasc Surg 2004;127:1350-60.
- Maxfield RA: New and emerging minimally invasive techniques for lung volume reduction. Chest 2004;125:777-83.



