ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Ascending-To-Descending Aortic Bypass For Coarctation of the Aorta

Patient Selection
Several surgical approaches have been described for the management of adult patients with acquired heart disease and paraductal coarctation of the aorta. A staged procedure using both median sternotomy and left thoracotomy, a catheter-based intervention of the coarctation combined with sternotomy, and single-stage simultaneous correction of both lesions via sternotomy are some options.
Preoperative Evaluation
The diagnosis and hemodynamic significance of the aortic coarctation should be confirmed prior to surgery. Thoracic aortogram with pressure gradient measurement across the coarctation gives a complete and reliable assessment of the anatomy and severity of the lesion. Computerized tomography (CT) angiogram or magnetic resonance angiography (MRA) are non-invasive alternatives to define the anatomy and severity of the coarctation, and is our preference. A gradient of 30 mmHg or greater is considered significant and an indication for correction. Surgery is also indicated for patients who present with symptoms of worsening fatigue, dyspnea on exertion, and severe hypertension requiring escalating doses of anti-hypertensive medications. Additional preoperative evaluation should include either transthoracic or transesophageal echocardiogram to determine ventricular function and the presence of hypertrophy, and to interrogate the aortic valve for pathology. Up to 40% of patients with coarctation will have a bicuspid aortic valve. Cardiac catheterization with coronary angiogram should be performed for patients at risk for coronary artery disease. A complete evaluation of the thoracic aorta is critical in identifying the presence of significant calcific atherosclerotic disease in the ascending and descending thoracic aorta, which may preclude the approach. We use non-contrast CT for this assessment.
Operative Steps
Our preference is to use intraoperative transesophageal echocardiography (TEE) to evaulate valvular pathology and confirm the absence of ascending and descending aortic atheroma. In addition, TEE probe is left in place in the transgastric position to assist in protection of the esophagus during the posterior pericardial dissection. After standard median sternotomy, the ascending aorta is completely mobilized to the aortic arch. Arterial cannulation is performed high in the aortic arch below the innominate artery. The posterior pericardial reflection is opened to the right of the inferior vena cava and the oblique sinus is developed. Cardiopulmonary bypass is implemented at 2.2-2.4 L/Min/m2, with systemic cooling to a temperature of 32 degrees C. Both antegrade and retrograde cardioplegia are used to provide optimal myocardial protection, as most patients have significant ventricular hypertrophy. Topical ice hypothermia aids in myocardial protection.
Following cardioplegic arrest, the apex is then elevated out of the pericardial well to expose the posterior pericardium. The descending thoracic aorta and the esophagus are palpated. The pericardium is opened in a longitudinal fashion, from the diaphragmatic recess to just inferior to the pericardial reflection onto the left inferior pulmonary vein. This will expose the descending thoracic aorta where the distal anastomosis will be created. Care is taken not to injure the esophagus on the right, (aided by palpation of the TEE probe), and to expose enough length of aorta to allow for comfortable placement of a Satinsky or other partial occlusion vascular clamp.
A graft sizer is then used to estimate the diameter of synthetic graft used, based on the size of the descending aorta. Usually an 18 mm to 22 mm vascular graft will suffice for most adults. The length of graft needed may be estimated using umbilical tape, routing the tape behind the IVC through the oblique sinus, and around the right atrium to the right lateral ascending aorta. A 26 to 30 cm graft length is usually adequate in most adults. The graft should be beveled slightly to allow for a some angulation superiorly, in order to route the graft behind the inferior vena cava and anterior to the inferior pulmonary vein.
The partial occlusion clamp is then applied to the anterior wall of the exposed descending thoracic aorta. A longitudonal aortotomy is made with Potts scissors, and an end-to-side anastomosis is constructed using 4-0 polypropolene running suture. This is technically the most difficult part of the operation, and careful and accurate placement of the sutures is essential for optimal hemostasis (Figures 1, 2). After routing the graft behind the IVC as described, a clamp is placed onto the midportion of the graft and the partial occlusion clamp is removed. Adequate hemostasis is ensured at the anastomosis, and the heart is lowered back into its normal position.
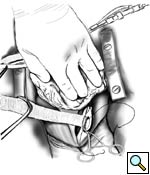 |
 |
| Figure 1 | Figure 2 |
At this point, other concomitant cardiac procedures may be performed, in the usual fashion.
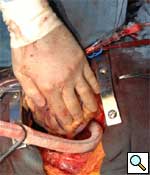
 |
| Figure 3 |
The proximal graft anastomosis is then performed with the heart arrested. This may be done after the crossclamp is removed, with the heart beating, but as stated below, the sequence as we describe (with the aorta filled) lends itself to a more accurate routing of the graft from the aorta, around the right margin of the heart. From the distal anastomosis, the graft is routed through the oblique sinus as described above, to a point several centimeters proximal to the crossclamp.
Longitudonal aortotomy is made at this point on the right lateral wall of the ascending aorta (identified while the aorta is filled with antegrade cardioplegia). The graft is beveled and tailored to adequate length and orientation, to provide a smooth course around the right margin of the heart, taking care to ensure the graft does not compress the right atrium. A running anastomosis is created with 4-0 polypropolene suture (Figure 3), and the aortic root is deaired through the anastomosis prior to removal of the aortic crossclamp. Final graft configuration is illustrated in Figure 4.
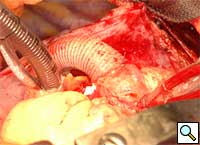
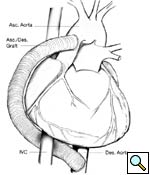 |
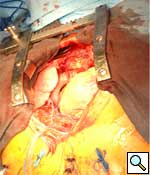 |
| Figure 4 | Figure 5 |
Following separation from cardiopulmonary bypass, transesophageal echocardiography is performed, to confirm adequate antegrade flow through the graft and to confirm the absence of intracardiac air. Photograph of graft in position is shown in Figure 5.
Postoperative Management
Paradoxical Hypertension. Some patients may experience a paradoxical hypertensive episode up to 72 hours after coarctation repair or bypass, thought to be due to increased aortic and carotid baroreceptor sensitivity (‘early’ phase, up to 24 hours postop), and increased circulating renin and angiotensin (‘late’ phase, 24-72 hours postop). This should be treated aggressively with intravenous sodium nitroprusside or beta-blocker, supplemented by early use of intravenous enalaprilat. ACE1 inhibitors should be continued by mouth once taking oral medications. Up to half of patients may experience this syndrome, which usually resolves in 24-48 hours.
Mesenteric Arteritis. The ‘late’ phase hypertensive episode may also lead to mesenteric arteritis secondary to inflammation, with possible small bowel necrosis. This syndrome may present with a wide range of symptoms, from mild abdominal pain to severe pain with fever, ileus, and GI bleeding. As a result, its reported incidence has varied from 7 to 28% of postoperative patients [1].
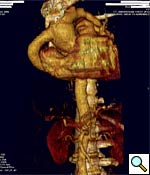 |
| Figure 6 |
Blood Pressure Control. Many patients will see either a resolution of their hypertension, or some degree of lessening in their required medications to control their blood pressure [2, 3].
Aspirin therapy alone is used after surgery, unless there is some other indication for coumadin anticoagulation.
Patients should be followed longitudonally with CT angiogram or MRA, to evaluated long term graft patency. Our first evaluation is usually 6 months postoperatively (Figure 6).
Tips & Pitfalls
- Always perform the distal graft anastomosis with the heart arrested and decompressed. This provides a bloodless field in which to sew this crucial anastomosis most accurately. Bleeding from this area later can be very difficult to control.
- It is critical to route the graft around the right margin of the heart without compressing the right atrium or right ventricle. This is best done by routing the graft behind the inferior vena cava and anterior to the inferior pulmonary vein. This course also ensures that the graft remains in a posterior location and to the right of the sternum, making future secondary sternotomy less hazardous.
- Size the length of the graft with the heart and aorta full, using an umbilical tape. This enables the surgeon to measure and tailor the graft accurately.
- Hemodynamic instability may occur after weaning from cardiopulmonary bypass. This is due to the decreased afterload from ‘runoff’ in the new conduit, and abnormal aortic elasticity in patients with coarcation [4]. Liberal use of vasoconstrictors to increase afterload and stabilize blood pressure is often required in the early post-cardiopulmonary bypass period.
DISCUSSION
The single-stage approach with ascending-to-descending aorta bypass was originally described by Vijayanagar [5]. This technique combined aortic valve replacement with a bypass graft from the ascending aorta to the retrocardiac descending thoracic aorta, using a posterior pericardial exposure of the descending thoracic aorta for the distal anastomosis, and the proximal anastomosis on the left side of the ascending aorta, routing the graft around the left side of the heart. Other authors have described a similar posterior pericardial approach, with modification to the technique by routing the graft around the right margin of the heart and constructing the proximal anastomosis on the right aspect of the ascending aorta [6, 7]. This technique kept the graft away from the midline, in a safer position in the chest for secondary sternotomy. In 2001, Connolly, et al, published a series of 18 patients treated with this technique, for indications of either concomitant cardiac procedure or complex coarctation/ recoarctation. They reported no surgical mortality, no paraplegia, no graft-related complications, and a significant improvement in systolic blood pressure after surgery [8].
In our opinion, the single-stage approach via median sternotomy provides several distinct advantages. This technique avoids the complications of anatomic repair with recoarctation (5-30% risk) [8], and of interventional repair with a 14-18% risk of acute complications, including aortic rupture/ dissection [9, 10]. It also avoids or minimizes the risk of paraplegia, and avoids the need for an additional surgical sternotomy or thoracotomy with a staged procedure. Patients who have significant atherosclerotic disease of the descending thoracic aorta should not be treated with this procedure. Although selection of the appropriate surgical approach to these patients should be individualized, the single-stage ascending-to-descending aorta bypass with concomitant cardiac procedure provides good results and a practical, safe alternative for treatment of this lesion set.
References
- Tawes RL Jr, Bull JC, Roe BB. Hypertension and abdominal pain after resection of aortic coarctation. Ann Surg 1970;171:409-12.
- Bouchart F, Dubar A, Tabley A, Litzler PY, Haas-Hubscher C, Redonnet M, Bessou, JP, Soyer R. Coarcation of the aorta in adults: surgical results and long-term follow-up. Ann Thorac Surg 2000;70:1483-88.
- Wells WJ, Prendergast TW, Berdjis F, Brandl D, Lange PE, Hetzer R, Starnes VA. Repair of coarctation of the aorta in adults: the fate of systolic hypertension. Ann Thorac Surg 1996;61:1168-71.
- Pethig K, Wahlers T, Tager S, Borst HG. Perioperative complications in combined aortic valve replacement and extraanatomic ascending-descending bypass. Ann Thorac Surg 1996;61:1724-26.
- Vijayanagar R, Natarajan P, Eckstein PF, Bognolo DA, Toole JC. Aortic valvular insufficiency and postductal aortic coarctation in the adult. Combined surgical management through median sternotomy: a new surgical approach. J Thorac Cardiovasc Surg 1980;79:266-68.
- Izhar U, Schaff HV, Mullany CJ, Daly RC, Orszulak TA. Posterior pericardial approach for ascending aorta-to-descending aorta bypass through a median sternotomy. Ann Thorac Surg 2000;70:31-37.
- Morris RJ, Samuels LE, Brockman SK. Total simultaneous repair of coarctation and intracardiac pathology in adult patients. Ann Thorac Surg 1998;65:1698-702.
- Connolly HM, Schaff HV, Izhar U, Dearani JA, Warnes CA, Orszulak TA. Posterior pericardial ascending-to-descending aortic bypass: an alternative surgical approach for complex coarcation of the aorta. Circulation 2001;104;133-37.
- Forbes TJ, Garekar S, Amin Z, et al. Procedural results and acute complications in stenting native and recurrent coarctation of the aorta in patients over 4 years of age: a multi-instititutional study. Catheter Cardiovasc Interv 2007;70:276-85.
- Hamdan MA, Maheshwari S, Fahey JT, Hellenbrand WE. Endovascular stents for coarcation of the aorta: initial results and intermediate-term follow-up. J Am Coll Cardiol 2001;38:1518-23.




Comments