ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Atresia of the Aortic Arch: Repair by Single-Stage Extra-Anatomic Ascending-to-Descending Aortic Bypass Graft Surgery
Escobar, Alejandro; Marin, Mateo; Turizo, Juan; Meza, Rafael (2017): Single-stage Extra-anatomic Ascending-to-descending Aortic Bypass Graft Surgery for Repair of Atresia of the Aortic Arch.
CTSNet, Inc.. https://doi.org/10.25373/ctsnet.5119924
Retrieved: 20:21, Jul 18, 2017 (GMT)
Introduction
Atresia of the aortic arch is a rare condition among adults. It must be anatomically differentiated from an interrupted aortic arch (IAA), which is characterized by a complete lack of anatomic continuity between the transverse aortic arch or isthmus and the descending thoracic aorta (1-5). In atresia of the aortic arch, continuity between the segments is achieved by an imperforate, fibrous cord of variable length, although the hemodynamic and physiological effects may be identical in both entities (1-3). In this case report, the authors describe the history of a 45-year-old man with symptomatic heart failure secondary to atresia of the aortic arch and associated coronary artery disease.
Case report
In 2017, a 45-year-old man presented at the authors’ hospital because of dyspnea on exertion, swelling of his legs, and orthopnea. These symptoms had begun several months earlier and were accompanied by recent angina pectoris. On physical examination, peripheral pulses were palpable over the carotid arteries and in the upper limbs, but inguinal pulses were almost imperceptible. The blood pressure was 173/102 mm Hg in the left arm, 174/101 mm Hg in the right arm, and 66/51 mm Hg in the right leg. Echocardiography revealed segmental contractility disorders, bicuspid aortic valve with neither stenosis nor regurgitation, normal diameter of the ascending aorta, and aortic coarctation distal to the origin of the left subclavian artery.
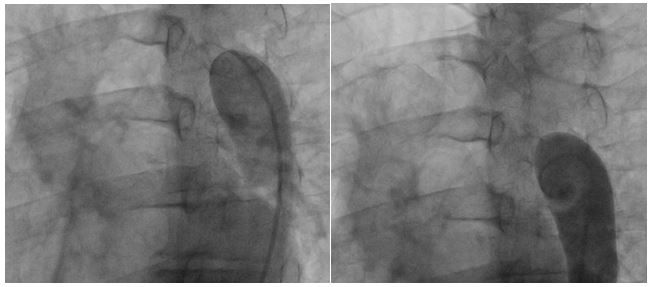
Femoral access cardiac catheterization showed complete occlusion of the thoracic descendent aorta distal to the left subclavian artery, without passage of the wire through the occlusion zone and with an 85 mm Hg gradient (Fig. 1A and 1B). Radial access cardiac catheterization showed significant proximal stenosis of the left anterior descending artery, circumflex artery, and right coronary artery.
Figure 1A and 1B: Thoracic aortogram. Aortic occlusion is evident.
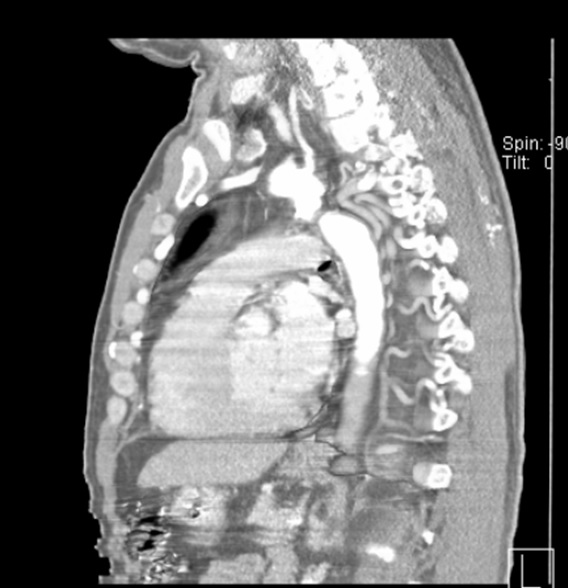
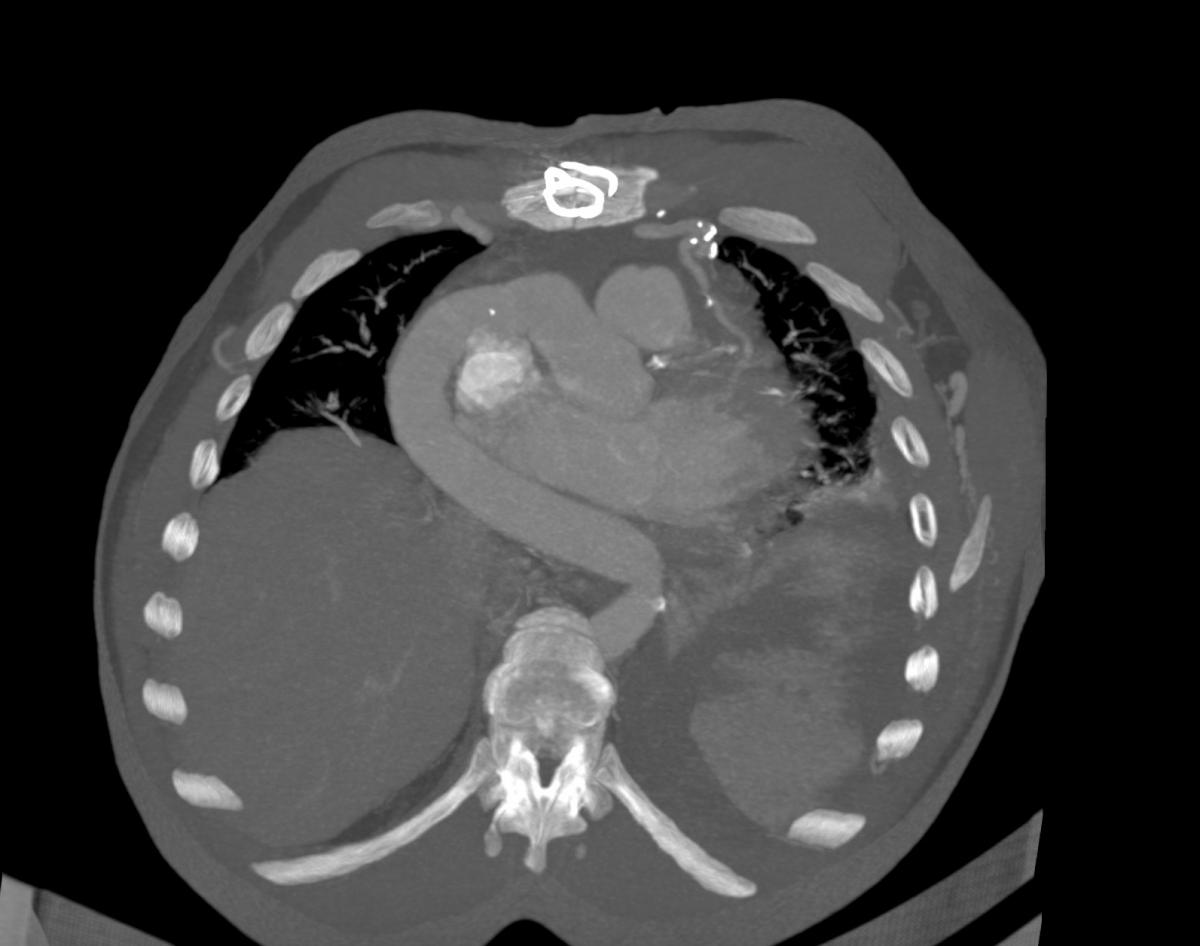
Chest computed tomography (CT) angiography revealed atresia of the aortic arch distal to the origin of the left subclavian artery. The descending thoracic aorta was dilated distal to the occlusion zone and it was supplied by prominent collateral circulation from internal mammary arteries, intercostal arteries, and the vertebrobasilar system (Fig. 2). Cerebral magnetic resonance imaging (MRI) demonstrated diffuse lacunar infarcts with no evidence of berry aneurysms in the circle of Willis.
A single-stage operation was performed through a midline sternotomy. Arterial cannulation was on the ascending aorta and the venous cannula was inserted into the right atrial appendage for venous return. Differential blood pressure was closely monitored during surgery, with radial and femoral arterial lines to confirm accurate distal perfusion.
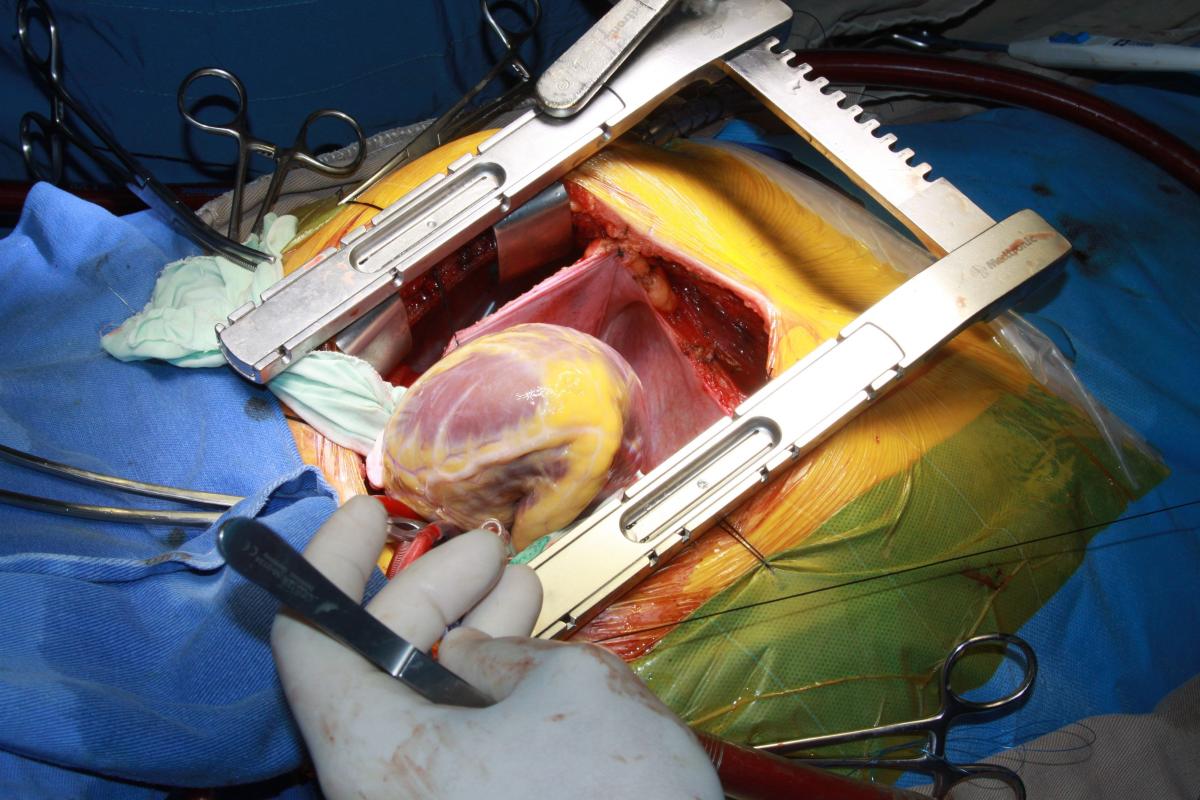
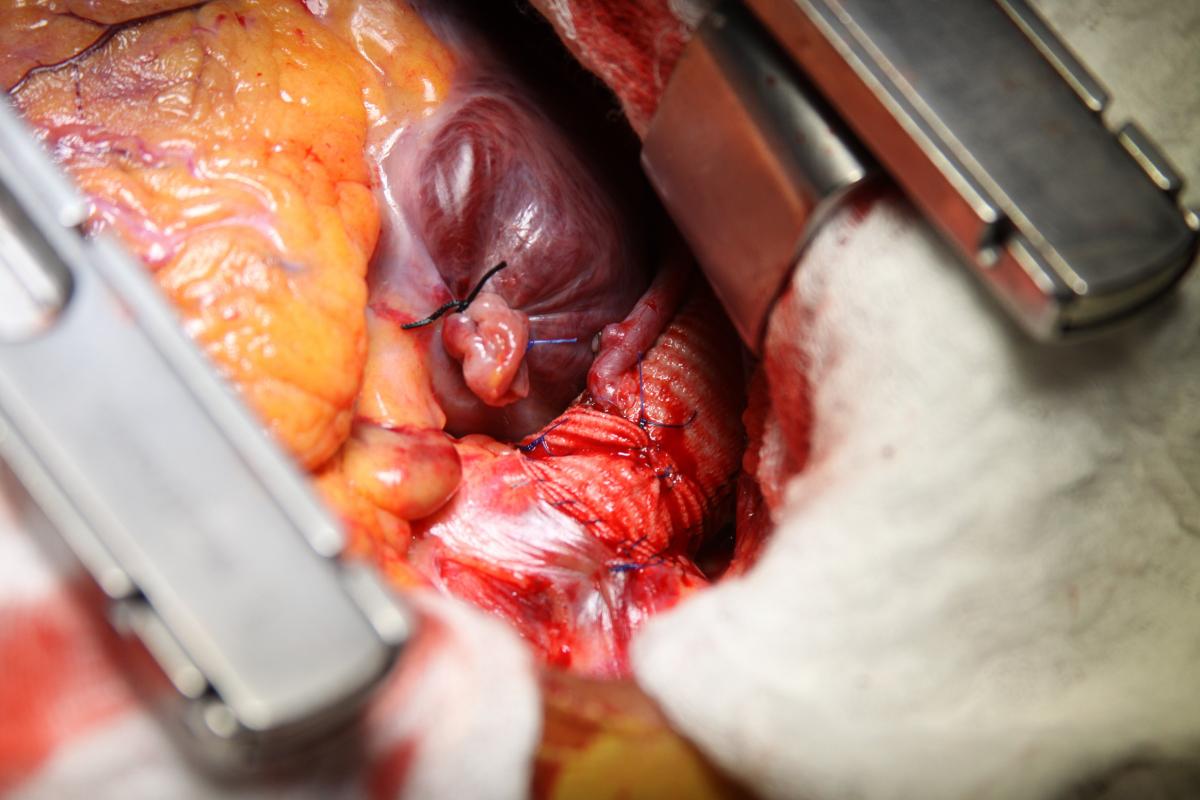
The left internal mammary artery (LIMA) was dissected in a skeletonized fashion. Flow was adequate, although the LIMA was found to have significant atherosclerosis. The aorta was cross-clamped, and antegrade Del Nido cardioplegia was delivered through the aortic root to induce diastolic cardiac arrest. Cardiopulmonary bypass (CPB) was then initiated under moderate hypothermia. Distal saphenous anastomoses of the circumflex artery and right coronary artery were made, and the cardiac apex was then elevated out of the pericardial sac to expose the posterior pericardium (Fig. 3). Care was taken not to injure the esophagus on the right by palpation of a nasogastric tube previously positioned into the esophagus. The pericardium was opened in a longitudinal fashion, exposing the descending thoracic aorta (Fig. 4).
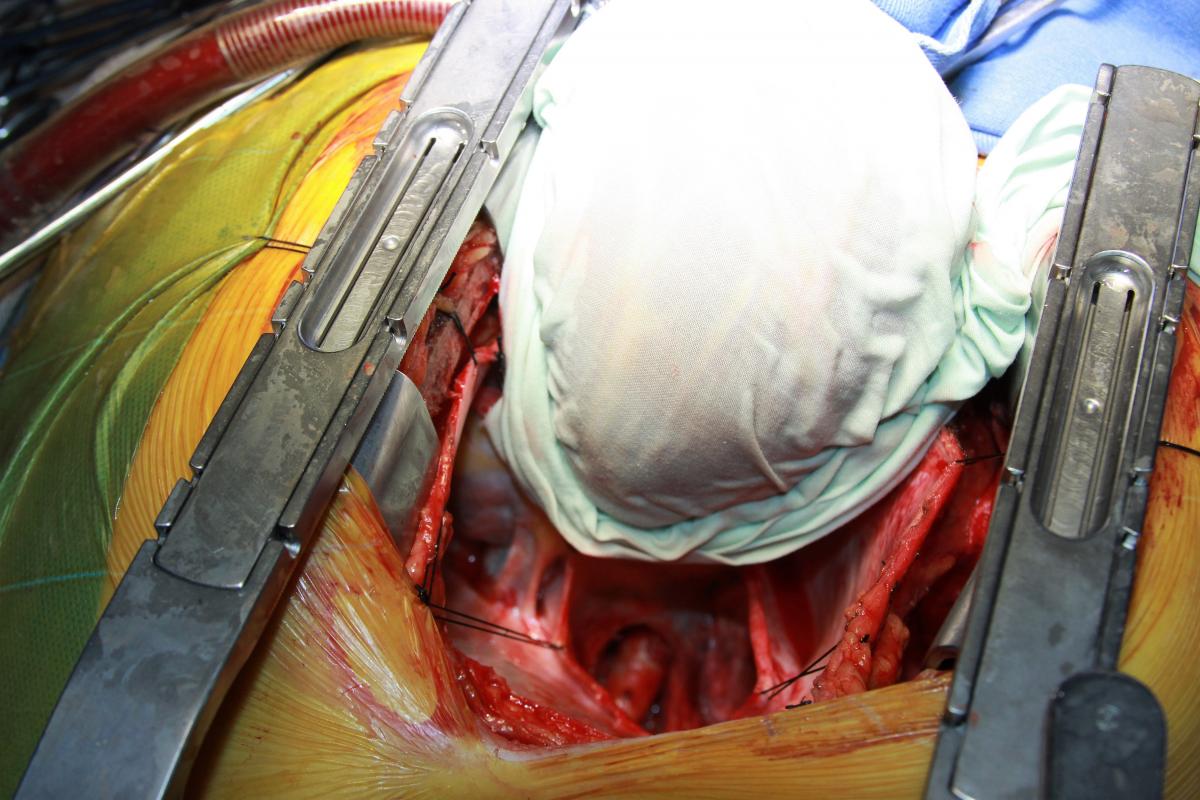
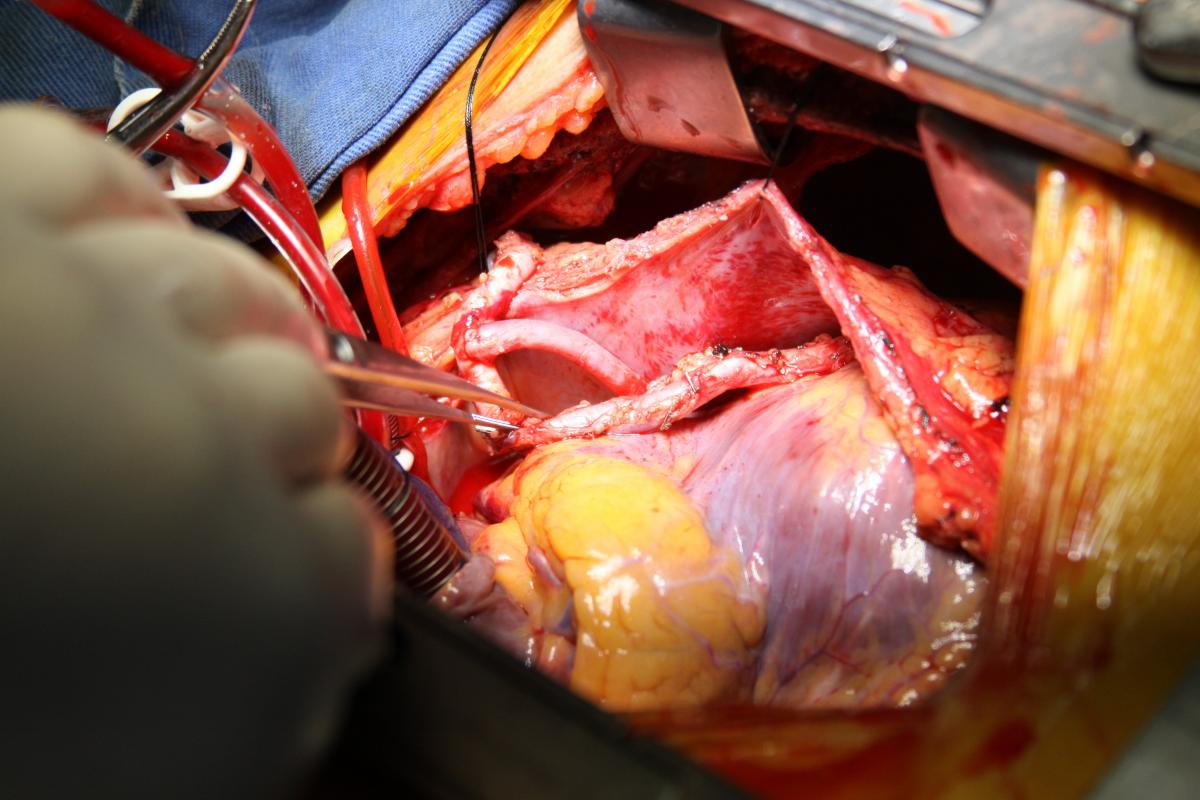
Under proximal and distal cross-clamping, a longitudinal aortotomy was made and a 20 mm diameter Dacron graft was anastomosed end-to-side to the descending thoracic aorta with a running 4-0 polypropylene suture. The diameter of the graft was selected according to the measured descendent aortic size and in accordance with the literature recommendations. The graft was routed behind the inferior vena cava through the oblique sinus, around the right atrium, and above the right pulmonary veins to the right lateral aspect of the ascending aorta (Fig. 5). The proximal portion of the graft was then anastomosed to the ascending aorta, in end-to-side fashion with a running 4-0 polypropylene suture (Fig. 6).
Finally, an anastomosis with the LIMA to the anterior descending artery was made. The circumflex artery proximal venous anastomosis was created as a Y graft to the LIMA (Fig. 7). Proximal right coronary artery venous anastomosis was then performed on the anterior surface of the Dacron graft, near the proximal end-to-side anastomosis to the ascending aorta (Fig. 5-6).
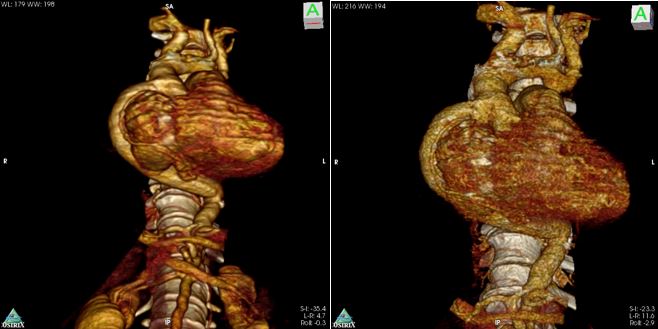
There were no perioperative complications with minimal postoperative bleeding. Extubation occurred 24 hours after surgery. The patient was transferred from the intensive care unit to the intermediate care unit on postoperative day two, and he was finally discharged from the hospital at postoperative day six. Control chest CT angiography revealed a patent graft and good distal flow (Fig. 8 and Fig. 9). The patient recovered uneventfully and continues doing well.
Discussion
Atresia of the aortic arch is a rare condition among adults. Its clinical presentation varies from absence of symptoms to headache, malaise, hypertension, claudication, differential blood pressure between the arms and legs, limb swelling, and congestive heart failure. The presence of collateral circulation is the only means of survival (6); however, collateral vessels are subject to atherosclerosis, which can lead to other challenging problems like concomitant coronary artery disease (7). A bicuspid aortic valve is commonly associated with atresia of the aortic arch, IAA, and coarctation of the aorta (8).
Atresia of the aortic arch should be differentiated from IAA, as the latter is characterized by a complete lack of anatomic continuity between the transverse aortic arch or isthmus and the descending thoracic aorta (1-5). If the IAA is left untreated, 90% of the affected infants die at a median age of 4 days (9). Blood flow to the descending aorta depends on the patency of the ductus arteriosus and, because it is almost always widely patent at birth, a collateral circulation does not develop and death occurs when the ductus closes soon after birth. Large ventricular septal defect is nearly always present (10).
Figure 8: Postoperative chest CT angiogram 3D reconstruction.
Although echocardiography has some limitations in evaluating the aortic arch and the descending aorta, it is the technique of choice for detecting concomitant cardiac abnormalities. In addition, CT and MRI are useful as combined diagnostic imaging methods (11). Aortography may be also useful. Berry aneurysms in the Willis circle should always be sought with cerebral imaging (12).
Atresia of the aortic arch in adults poses a surgical challenge with or without associated intracardiac disease. Extra-anatomical aortic bypass via median sternotomy is a safe and less invasive technique for repairing atresia of the aortic arch, IAA, and aortic coarctation. The extra-anatomic bypass is also the preferred technique to repair complex coarctations and recoarctation in patients already operated in early childhood. It can be performed with low morbidity and mortality (13, 14).
This technique via the posterior pericardium is also an excellent single-stage approach for this complex congenital disease with other concomitant surgical cardiovascular disorders. Patients with atresia of the aortic arch (also coarctation or IAA) and associated cardiac defects that are corrected at two separate surgical times may have worse outcomes, because each separate procedure has its own inherent risks and costs (15).
The first disadvantage of this procedure is that it does not provide good access to the descending aorta, making it difficult to control bleeding from the distal anastomosis. Secondly, CPB assistance is always required because the heart has to be retracted cephalad for exposure of the descendent aorta posterior to the pericardium, which will affect hemodynamics (16). Special attention should be paid to the Dacron graft, because it could collapse adjacent structures such as the right atrium, right pulmonary veins, and inferior vena cava.
This patient underwent coronary artery and ascending-to-descending aorta bypass grafting in one single stage with successful results. Standard ascending aorta cannulation was performed with narrow surveillance of differential blood pressure in the upper and lower limbs. Femoral arterial cannulation for CPB was considered unnecessary because the authors estimated collateral circulation was good enough to provide appropriate distal perfusion.
In conclusion, atresia of the aortic arch is rarely encountered in adult patients and may coexist with other surgical heart conditions. It should be distinguished from IAA and aortic coarctation. The single-stage extra-anatomic ascending-to-descending aortic bypass graft surgery is a procedure with a low risk of morbidity and mortality for this congenital malformation. When performed via midline sternotomy, correction of other surgical heart pathologies like coronary artery disease may be feasible.
References
- Van Praagh R, Bernhard WF, Rosenthal A, Parisi LF, Fyler DC. Interrupted aortic arch: surgical treatment. Am J Cardiol 1971; 27: 200–11.
- Anderson RH, Macartney FJ, Shinebourne EA, Tynan M. Interruption of the aortic arch. In: Paediatric Cardiology. Edinburgh: Churchill Livingstone, 1981: 1087–121.
- Freedom RM, Mawson J,Yoo S-J, Benson LN. Congenital Heart Disease: Textbook of Angiocardiography, Vols 1 & 2. Armonk, NY: Futura, 1997: 1432.
- Celoria GC, Patton RB. Congenital absence of the aortic arch. Am Heart J 1959; 58: 407–13.
- Fyler DC. Interrupted aortic arch. In: Fyler DC, ed. Nadas’ Pediatric Cardiology. St Louis, MO: Mosby-Year Book, 1992;549–53.
- Tajdini M, et al. Asymptomatic Interrupted Aortic Arch, Severe Tricuspid Regurgitation, and Bicuspid Aortic Valve in a 76-Year-Old Woman. Tex Heart Inst J 1984;11(3):250-9.
- Kreiger KH, Spencer FC. Is paraplegia after repair of coarctation of the aorta due principally to distal hypotension during aortic cross-clamping? Surgery 1985;97:2-7.
- Tawes RL Jr, Berry CL, Aberdeen E. Congenital bicuspid aortic valves associated with coarctation of the aorta in children. Br Heart J. 1969 Jan;31(1):127-8.
- Collins-Nakai RL, Dick M, Parisi-Buckley L, Fyler DC, Castaneda AR. Interrupted aortic arch in infancy. J Pediatr 1976;88:959-62.
- Jonas RA1, Quaegebeur JM, Kirklin JW, Blackstone EH, Daicoff G. Outcomes in patients with interrupted aortic arch and ventricular septal defect. A multinstitutional study. Congenital Heart Surgeons Society. J Thorac Cardiovasc Surg. 1994 Apr;107(4):1099-109
- Reardon MJ, Hallman GL, Cooley DA. Interrupted aortic arch: brief review and summary of an eighteen-year experience.
- Shearer WT, Rutman JY, Weinberg WA, Goldring D. Coarctation of the aorta and cerebrovascular accident: a proposal for early corrective surgery. J Pediatr. 1970;77:1004–1009.
- Heidi M. Connolly, MD; Hartzell V. Schaff, MD; Uzi Izhar, MD; Joseph A. Dearani, MD; Carole A. Warnes, MD; Thomas A. Orszulak, MD. Posterior Pericardial Ascending-to-Descending Aortic Bypass An Alternative Surgical Approach for Complex Coarctation of the Aorta Circulation. 2001;104[suppl 1]:I-133-I-137
- Almeida de Oliveira S, et al. Extraanatomic Aortic Bypass for Repair of Aortic Arch Coarctation via Sternotomy: Midterm Clinical and Magnetic Resonance Imaging Results. Ann Thorac Surg 2003;76:1962– 6
- Wang et al. Treatment of complex coarctation and coarctation with cardiac lesions using extra-anatomic aortic bypass. J Vasc Surg 2010;51:1203-8
- Brink J, et al. Complications of Extra-Anatomic Aortic Bypass for Complex Coarctation and Aortic Arch Hypoplasia. Ann Thorac Surg 2013;95:676–81





Comments