ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
The Recurrent Laryngeal Nerves and the Thoracic Surgeon
Amer, Khalid (2017): The Recurrent Laryngeal Nerves and the Thoracic Surgeon.
CTSNet, Inc. https://doi.org/10.25373/ctsnet.5345176
Retrieved: 20:16, Sep 19, 2017 (GMT)
Introduction
Many thoracic surgeons are terrified to come anywhere near the recurrent laryngeal nerve (RLN), especially on the left side. The reason for this fear is the dreaded complication of damaging the nerve and causing loss of voice, among other serious complications. RLN palsy ranks among the leading reasons for medicolegal litigation of surgeons because of its attendant reduction in quality of life. It is not surprising, therefore, that thoracic surgeons can be timid and self-assuring in their reasoning that ‘the best way of avoiding injury to the RLN is not to look for it’. Unfortunately this adage has gained credit through long use and the lack of clear descriptive anatomy in the medical literature targeting the needs of the thoracic surgeon. It is the purpose of this publication to dissipate all the myth around the anatomy of RLN, and encourage its deliberate exposure as a first step towards safety. In the author’s unit, this approach has virtually eliminated the risk of injuring the nerves during mediastinal nodal dissection. It has to be said that the use of bipolar diathermy devices in video assisted thoracoscopic surgery (VATS) played a major role in safe dissection around the RLN.
Evolution
The course of RLN in mammals is thought to be an extreme detour, about 4.6 meters (15 ft) in the case of the giraffe [1]. The nerve avoids the direct route between brain and throat and instead descends into the chest, then makes a U-turn around the aorta, and returns to the larynx. That makes it seven times longer than it needs to be. This unnecessary stretch down to the chest, and the sudden change of direction to ascend to the larynx is considered by evolution scientists as ‘evidence of poor design’. But is the model defective? This remains debatable [2]. Apart from the motor nerves to the larynx, the recurrent laryngeal nerves supply a plethora of autonomic and sensory nerves on its way up and these must have some value assigned to their sensory input and autonomic reflexes.
Function
The RLNs supply all the intrinsic muscles of the larynx (voice box), with the exception of the cricothyroid muscles (CT). The latter is supplied by the superior laryngeal nerve (SLN), a branch of the Vagus. The vocal cords are made of an intrinsic muscle called thyroarytenoid (TA) wrapped inside a thin layer of mucosa. The posterior cricoarytenoid (PCA) muscles open or abduct the vocal cords, whereas the lateral cricoarytenoid muscles (LCA) adduct or close the vocal cords. The RLN also supplies laryngeal sensation below the vocal cords, and the superior laryngeal nerve supplies sensation above the cords. The TA muscle does not work in isolation to control the voice, but in harmony with all other intrinsic muscles. Voice control is quite a sophisticated physiology that is controlled by the vagus nerve and RLN, both arising directly from the brain rather than segmentally from the spinal cord. Unilateral trauma to the RLN causes ipsilateral vocal cord paralysis which lies in the paramedian or abducted position (slightly off the median line). The paramedian position results in a normal but weak voice. Alternatively the voice could be hoarse with ineffective cough and choking fits. Patients with recognized palsy should be referred to speech and language therapy services. Unilateral paralysis can improve spontaneously due to compensation by the normal cord which crosses the midline to meet the paralysed one. Generally no treatment is required. Usually 6 months is an adequate time to wait for spontaneous recovery. Failure to improve warrants referral to an ear, nose, and throat (ENT) specialist. Bilateral injuries to both RL nerves cause stridor and complete loss of voice, as well as difficulty with spontaneous breathing. The airways are functionally obstructed by the two paralysed cords, which lie in a median or paramedian position due to unopposed action of CT muscles. An urgent tracheostomy might be needed to allow spontaneous breathing.
Injury
Injury of the RLN can occur from infection, inflammation, trauma, surgery, or malignant involvement (thyroid, parathyroid, cervical esophagus, tracheal lung, lymph nodes). Thyroid and parathyroid surgery carry the highest risk to permanent RLN palsy (3.5%) [3]. Cardiac operations are also common causes of palsy, especially aortic aneurysms and ductus arteriosus surgery. Recent development of routine systematic mediastinal nodal dissection (SND) during surgery for non-small cell lung cancer (NSCLC) has seen a surge in the incidence of RLN palsy. All patients undergoing SND should be made aware of the possibility of RLN palsy and should be consented appropriately. It would be wise to omit dissection of left 4L nodal stations in a soprano singer. Surgery for mediastinal tumors and thymic masses overhanging the aortic arch pose a high risk for RLN palsy. Likewise, these patients must be appropriately consented for this complication. Vocal cord palsy in the immediate postoperative period is particularly troublesome. It leads to breathing problems, choking fits, a weak cough that can lead to retention of secretions, aspiration, and lung consolidation. In patients with critically marginal lung reserves, this could be the last straw leading to a lethal outcome. Escalation of care to a high dependency unit or even an intensive care unit and the need for tracheostomy and mechanical ventilation might be indicated.
The Right Recurrent Laryngeal Nerve
The right RLN makes a quick entry and exit at the top of the right chest. To understand the course of the right RLN one has to understand the course of the right vagus nerve first. The latter originates from cell bodies in the nucleus ambiguus, the dorsal motor nucleus, and the inferior ganglion in the brain. It has central connections to the tractus solitarius and its peripheral distribution influences speech, swallowing, heart beating, gastrointestinal motility, digestion, taste, and sensations. The vagal trunk exits the base of the skull via the jugular foramen, lateral to the carotid artery. In its descent in the neck, it keeps this lateral relation to the carotid artery, contained within its sheath all the way down to the thoracic inlet. At the origin of the carotid artery, the nerve crosses lateral to the bifurcation of the brachiocephalic artery, and descends over the anterolateral part of the main stem trachea, from medial to lateral, proceeding under the arching part of vena azygos and then behind the hilum of the lung. In its infra-azygos course, it lies superficial to the esophagus and between the ascending part of the azygos vein and bronchus intermedius. When it reaches the lower third of the esophagus, it breaks up and mingles with branches from the left vagus nerve to form the anterior and posterior esophageal vagal plexuses. Throughout its course in the chest, the vagus nerve remains covered by the mediastinal pleura.
In the neck, the right RLN separates off the vagus nerve at a variable point in its course but remains within its sheath (two bananas in one skin). It descends parallel to the vagus nerve all the way to the origin of the right common carotid and subclavian arteries. These two arteries are the terminal divisions of the brachiocephalic artery. The RLN is closely tucked around the bifurcation of the brachiocephalic artery, separate from the vagus nerve at this location. Anatomy books describe this looping point as being around the origin of the right subclavian artery [4]. This description is not helpful, as the thoracic surgeon does not relate to the subclavian artery readily. Strictly speaking this is true in cadaveric specimens, yet the origin of the subclavian is not seen by the thoracic surgeon approaching nodes via the chest. It is therefore practical to describe the nerve as “looping around the distal bifurcation of the brachiocephalic artery”. The recurrent laryngeal nerve gets its name from this looping: re curr a Latin word meaning “running in the opposite direction” (to the vagus nerve from which it branches). In 0.5% - 1% of population, a nonrecurring right RLN exists. A nonrecurrent laryngeal nerve is rare but has been described in right-sided aortic arch or retropharyngeal left subclavian artery [5].
As mentioned above this is a quick entry and exit in and out of the chest. Behind the bifurcation, the right RLN re-enters the neck and heads towards the groove between the trachea and esophagus. It reaches the groove at the level of the thyroid cartilage. Because the nerve is literally tucked to the artery at the point of reflection, it is unusual for it to be damage by cutting or diathermy before causing some serious bleeding. The commonest account for iatrogenic injury would be inadvertent spray of energy (monopolar diathermy or ultrasonic devices) close to the bifurcation of the brachiocephalic artery. The thoracic surgeon should avoid diathermy in this area.
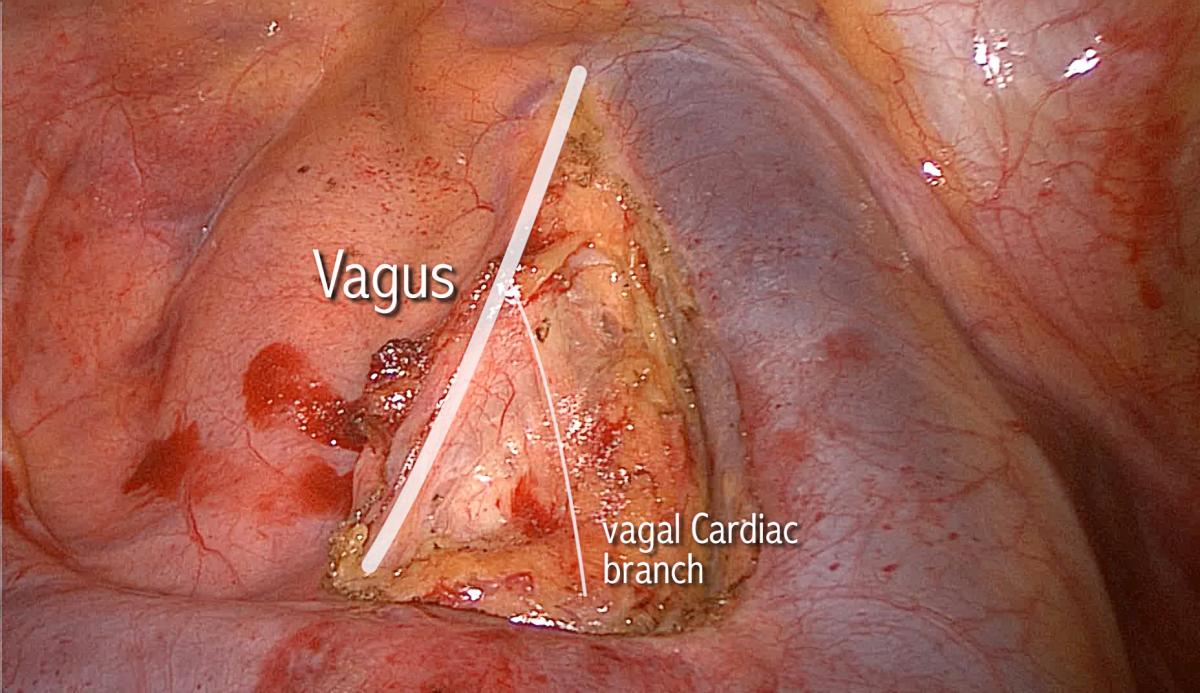
Just beyond the point of reflection, the vagus nerve behaves in a predictable organized chaotic manner. It is mainly the vagus nerve, but also the RLN, that contributes to a multitude of sensory and autonomic twigs to the surrounding structures such as the main trachea, the esophagus, cardiac branches to the arch of aorta and its major branches, and the nearby fibro-fatty-nodal block. Nodes, especially those of station 3p (retrotracheal), are trapped within these twigs, akin to fish trapped in a fisherman’s net. Interestingly, the same arrangement exists on the left side at the same point of reflection. There is no doubt that these chaotic sprouting twigs are the main cause of confusion surrounding the anatomy of the RLN. The motor laryngeal branch should remain the sole concern of the thoracic surgeon, and the remaining sensory twigs can be cut with impunity. One constant large branch of the vagus nerve is worth mentioning, the inferior cardiac branch, relatively large and seen in the superior triangle (made by the phrenic, vagus, and azygos vein) (Figure 1). It traverses the triangle from superolateral to inferomedial, heading towards the arch of aorta. This branch can be cut with impunity when dissecting nodes in stations 2-4R.
Early surgical exposure of the RLN safeguards against inadvertent injury. Bipolar devices are safe in that area and can be used to open the pleura investing the superior triangle. Only the pleura must be included within the jaws of the bipolar device. The vagus nerve should be followed upwards towards the apex until the brachiocephalic artery is seen. The laryngeal motor branch of the RLN will be found tucked to the artery at this point. Should the need arise, the sensory twigs are cut as distal as possible, close to their targets, to minimize spread of energy to the motor branch. The latter should be kept under vision at all times. The voice will not be affected if the motor branch is preserved. The secret to identifying what to cut and what not to cut lies in the large caliber of the motor nerve relative to the sensory twigs and to the fact that the motor branch is never seen more than 1-2 mm away from the artery.
The Left Recurrent Laryngeal Nerve
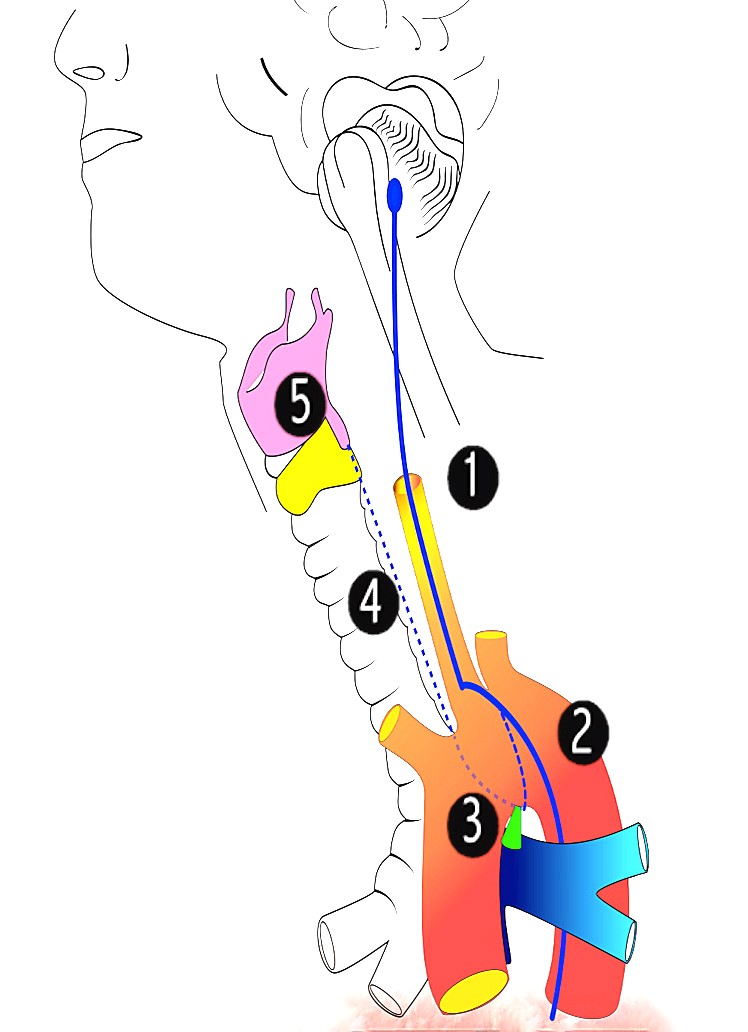
The left vagus nerve and left RLN can be described in 5 zones (Figure 2):
Zone 1: Descending part from base of skull to the aortic arch.
Zone 2: Over the aortic arch.
Zone 3: Under the aortic arch.
Zone 4: Ascending part above the aortic arch, within the tracheoesophageal groove.
Zone 5: Laryngeal part or target zone.
The left vagal trunk leaves the base of the skull and attains a position lateral to the carotid artery, similar to the right arrangement. This descending first part of the nerve carries both the descending vagus nerve and descending RLN (two bananas in one skin). When it reaches the origin of the carotid artery it climbs up the aortic arch, cursing below the superior intercostal vein, heading to the angle between ligamentum arteriosum and aortic arch (technically, the beginning of the descending aorta). Throughout its course in the chest, the vagus nerve remains covered by the mediastinal pleura.
Entering the thoracic inlet, the left vagal trunk runs between the left subclavian artery and phrenic nerve, and lateral to the left common carotid artery. Dissection for station 2L nodes in this position should avoid inadvertent transection of the vagal trunk, as this will result in RLN palsy. The ascending branch of the recurrent laryngeal (zone 4) is deeper than the left common carotid artery in this position, and is unlikely to be injured during harvesting of 2L nodes.
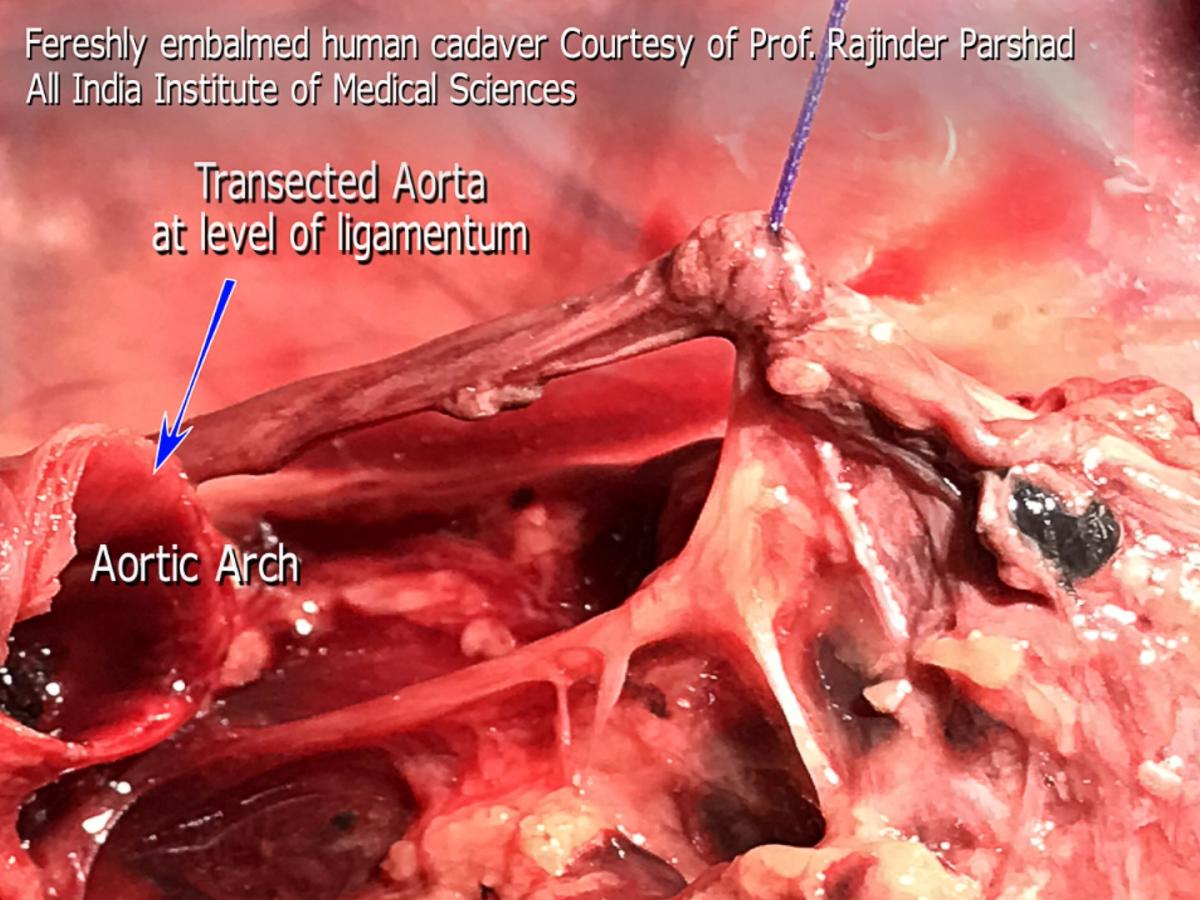
Figure 3. Freshly embalmed human cadaver. The aorta was transected at the origin of the ligamentum arteriosum to expose the motor branch in RLN zone 3.
In its second zone, the RLN usually splits from the vagus but runs very close and medial to it. Therefore, the thoracic surgeon might see one or two nerves side-by-side over the aortic arch. The caliber of the RLN is about 1 mm, or half that of the vagus nerve, but sizable enough to distinguish it from the sensory twigs that arise from it. At the point of origin of the ligamentum, the vagus nerve continues to descend over the back of the hilum, anterior to the descending aorta to reach a course lateral to the esophagus below the inferior pulmonary ligament. It is at this point of reflection at the ligamentum that the vagus nerve (as well as the RLN) gives sensory and autonomic nerve twigs to the surrounding structures (main trachea, esophagus, left main bronchus, cardiac branches, main pulmonary artery and nodal pack). This is a replica of the organized chaos seen on the right side. These twigs work like ropes that fix the vagus nerve and the RLN to the point of reflection around the ligamentum. They weave up a mesh that traps lymph nodes like trapped fish in a fisherman’s net (station 5 and 4L). The main motor branch is what the thoracic surgeon should be concerned about. The latter continues under the aortic arch into zone 3, retaining the same caliber seen over the arch, in contradistinction to the sensory twigs, which are considerably smaller in size. Similar to the right side arrangement, it is these sensory fibers that cause confusion of identification and fear of iatrogenic injury. Previously it was thought that disconnection of these sensory twigs is related to vocal chord palsy, which is not the case. The course of the motor part of the nerve as it disappears under the ligamentum has baffled surgeons for a long time. Very little is seen of the motor branch before it disappears under the aortic arch. The course of the nerve from the ligamentum to the point where the nerve reaches the tracheoesophageal groove is only few millimeters, but seems to be completely baffling to the thoracic surgeon. Contrary to common belief, the motor nerve does not ascend to the left hand side but to the right side to reach the groove (viewed from a thoracotomy incision or VATS lateral view). Figure 3 shows a cadaveric photograph taken from a freshly embalmed body, courtesy of Professor Rajinder Parshad, The All India Institute of Medical Sciences. It shows a transected aorta at the point of origin of the ligamentum arteriosum. The vagus nerve was slung using a suture material. The course of the motor RLN is shown clearly, and the difference in caliber compared to the sensory twigs is worth noting. Dissection for station 4L and 5L could be facilitated by deliberate cutting of these sensory twigs. Care should be taken to disconnect them using a bipolar device, close to their target destination, to minimize spread of energy to the motor branch. It is perfectly safe to do so, with no consequences or voice complications. The trick is to identify the motor branch and keep an eye on it at all times. Although not necessary, stapling and cutting the ligamentum arteriosum improves exposure of zone 3 of the nerve and facilitates nodal harvesting of station 4L, as well as making it possible to sample 4R precarinal (N3 nodes) anterior to the main trachea. Blind diathermy under the arch, or blind fishing for nodes with a ring forceps, even after identifying the recurring motor branch might disconnect the motor branch on its way to the trachea-esophageal groove. It is safe to dissect tissues and fibers to the left of the motor branch, but unsafe to do the same on the right side of the motor branch (Figure 4). There is a constant sensory twig to the left of the reflection point that supplies the pulmonary artery, which is a major cause for confusion and procrastination in decision taking. No nerve should be disconnected until the surgeon has decided beyond doubt that it is not the motor branch.
Zone 4 of the left RLN is very well known to the esophageal surgeons. Nodal dissection during VATS esophagectomy in the prone position gives exceptional views from the back of the trachea and esophagus. Safe nodal dissection dictates exposing the RLN to avoid iatrogenic injury in zone 4. The main motor nerve is seen to be sizable, and it gives small, hairline twigs right and left to the trachea and oeosphagus. This is helpful information to the general thoracic surgeon not exposed to such views. In zone 4, the RLN cannot help but annoy thyroid surgeons before reaching its destination.
Zone 5 is the domain of the ENT surgeons, as the nerve reaches its final destination, pierces the cricothyroid membrane, and starts doing what it was designed to do: giving motor branches to the vocal chords and sensory branches to the larynx.
Can injury to the RLN be avoided by monitoring the nerve activity during surgery? [6]
Intraoperative nerve monitoring (IONM) has been introduced to verify RLN functional integrity intraoperatively. This is a well-established practice in thyroid surgery, where high litigation rates are common. Electromyographical endotracheal tube systems are used to obtain electrical feedback from the vocal cards during the operation [7,8]. Detection rates and reliability of these systems is still a problem. There have been randomized controlled trials with regards to IONM in thyroid surgery, however none exist in thoracic surgery. These have not shown unequivocally that monitoring RLN prevents injury. Surgical units who have the means to IONM will have to use them regardless, as it will be a medico-legal requirement. In other words, surgeons are stuck with it; they have to demonstrate that ‘everything was done to safeguard against iatrogenic injury’. In our unit we witnessed a dramatic improvement since shifting practice to the usage of the articulating bipolar device Enseal, (Ethicon, Johnson and Johnson, Cincinnati, USA). We encountered this complication of RLN palsy in 3 out of 600 cases of mediastinal nodal dissection during lung resection for NSCLC. Two palsies occurred due to thermal injury following the use of monopolar diathermy close to the motor branch, and one was caused by inadvertent vagal trunk transection in zone 1 while harvesting 2L nodes.
Conclusion
The course of the right RLN is short, and the thoracic surgeon is unlikely to damage it. The left RLN anatomy is more complex and requires in-depth understanding. The thoracic surgeon is encouraged to expose the nerve early in nodal dissection to avoid damaging it. Absolute knowledge of the RLN anatomy is mandatory to thoracic surgical practice, and the author strongly believes that the best way to avoid injuring the RLN is to expose it and monitor its motor branch.
References
- Marshall Cavendish Corporation. Mammal Anatomy: An Illustrated Guide. Cavendish Square Publishing. 2010.
- Prothero D. 2008. Evolution: What the Fossils Say and Why It Matters. New York: Columbia University Press, 37-38.
- Misiolek M, Waler J, Namyslowski G, Kucharzewski M, Podwinski A, Czecior E. Recurrent laryngeal nerve palsy after thyroid cancer surgery: a laryngological and surgical problem. Eur Arch Otorhinolaryngol. 2001 Nov;258(9):460-462.
- Gray H. Anatomy of the Human Body: 5j. The Vagus Nerve. 2000. http://www.bartleby.com/107/205.html. Last accessed February 14, 2017.
- Stedman GW. Singular distribution of some of the nerves and arteries in the neck and the top of the thorax. Edin Med Surg J. 1823,19:564-565.
- Dralle H, Sekulla C, Lorenz K, Brauckhoff M, Machens A. German IONM Study Group Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg. 2008 Jul;32(7):1358-1366. doi: 10.1007/s00268-008-9483-2.
- Musholt J. Continuous Monitoring of the Recurrent Laryngeal Nerve. 2014. https://www.youtube.com/watch?v=sr0NsmMknZg. Last accessed February 14,2017.
- Birkholz T, Saalfrank-Schardt C, Irouschek A, Klein P, Albrecht S, Schmidt J. Comparison of two electromyographical endotracheal tube systems for intraoperative recurrent laryngeal nerve monitoring: reliability and side effects. Langenbecks Arch Surg. 2011 Dec;396(8): 1173–1179.





Comments