ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Superior Cavopulmonary Anastomosis: The Hemi-Fontan and Bidirectional Glenn
Patient Selection
The superior cavopulmonary anastomosis is the first of two operations used in a staged Fontan approach for definitive palliation of functionally single ventricle hearts. It was introduced into the palliative strategy to reduce the volume load on the hypertrophied single ventricle that pumps in parallel to the pulmonary and systemic circulations. By connecting the superior vena cava (SVC) to the pulmonary arteries and eliminating other sources of pulmonary blood flow, the ventricular output is directed exclusively to the systemic circulation and thus the volume work of the heart is reduced. There are two surgical techniques used to create this cavopulmonary connection prior to a completion Fontan, the bi-directional superior cavopulmonary anastomosis (bi-directional Glenn) and the hemi-Fontan.
Because the first stage of the Fontan is preparatory for the final one, the cavopulmonary anastomosis should facilitate construction of the completion Fontan. In the hemi-Fontan operation, the continuity of the SVC and right atrium is maintained when the SVC is connected to the right pulmonary artery. A dam of homograft tissue sewn across the superior cavoatrial junction prohibits blood flow into the right atrium from the SVC. At the time of the completion stage, the Fontan operation is performed as an atrial lateral tunnel technique in which the dam is excised and a gusset of polytetrafluoroethylene (PTFE) is sewn in place so as to subdivide the atrium such that the inferior vena caval (IVC) blood flow is directed into the pulmonary arteries, and pulmonary venous blood is channeled across the systemic atrioventricular valve(s). In contrast, constructing a bi-directional Glenn involves dividing the SVC at its junction with the right atrium, over-sewing the atrial end, and creating an end-to-side anastomosis between the SVC and the right pulmonary artery. In this case the Fontan completion is readily accomplished by sewing an extracardiac conduit in an end-to-end fashion to the IVC and in an end-to-side fashion to the pulmonary artery confluence or distal SVC.
Independent of the surgical technique used for the cavopulmonary anastomosis, preoperative catheterization and echocardiogram are important to provide hemodynamic and anatomic information. In particular the adequacy of the pulmonary artery size and architecture, the pulmonary vascular resistance (favorable if PVR < 2-3 Wood units/m2), and the function of the atrioventricular valves and systemic ventricle determine if the first stage of the Fontan palliation is feasible.
Operative Steps
The patient is positioned supine and an arterial line is placed for sampling blood gases and measuring blood pressure. Central venous access is not routinely obtained to avoid the risk of venous thrombosis. Following endotracheal intubation, the ambient temperature is decreased, ice bags are placed on the head, and a cooling blanket is used to cool the patient. A median sternotomy is performed and initially the aorta and right atrium are exposed. Heparin is administered to achieve an ACT suitable for cardiopulmonary bypass followed by cannulation of the ascending aorta and placement of a single atrial cannula for systemic venous drainage. Extracorporeal flow is established at 150cc/kg/min, and all systemic-to-pulmonary artery shunts are occluded. The perfusate is then cooled to approximately 16o C. While the patient is gradually cooled to both nasopharyngeal and esophageal temperatures of 17° C, the remainder of the dissection is performed to expose adequate ascending aorta for cross clamping and to carefully free the SVC, pulmonary arteries and right atrium. Once the desired temperature is reached, the aorta is clamped, cardiopulmonary bypass flow is temporarily stopped, and cold cardioplegia solution is infused to achieve an electromechanical cardiac arrest. Alternatively, the superior cavopulmonary anastomosis may be performed using moderate hypothermia and continuous circulatory support.
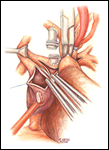
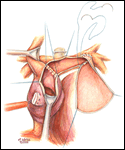
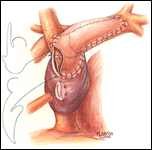
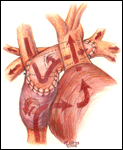
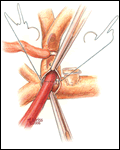
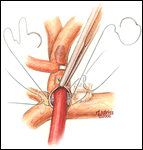
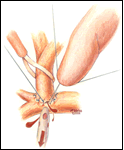
If a hemi-Fontan is performed (Figures 1-6), the venous cannula is removed. A right atriotomy is made beginning in the most superior portion of the atrium and extending onto the medial aspect of the SVC. The pulmonary artery confluence is incised anteriorly and the incision extended from a point medial to the right upper lobe branch leftward to a corresponding site on the left pulmonary artery. If there is antegrade flow across the pulmonary valve, the main pulmonary artery is transected and the valve over-sewn. The right SVC is joined to the right pulmonary artery in a side-to-side anastomosis. When a left SVC is present, it too is opened along its medial side, occluded at the cardiac end, and joined to the left pulmonary artery in a side-to-side anastomosis. A single patch of cryopreserved pulmonary artery homograft tissue is used to augment the branch pulmonary arteries anteriorly, to create a roof over the vena(e) cava(e)-to-pulmonary artery anastomosis(es), and to create a dam occluding flow from the right SVC into the ipsilateral atrium. Prior to creating the dam, the SVC-to-right atrium junction is enlarged to the caliber of the IVC. Following completion of all suture lines, the heart is filled with saline to displace air, the venous cannula replaced into the atrium, and cardiopulmonary bypass resumed.
After the heart is deaired, the cross clamp is removed, and the patient is rewarmed to a nasopharyngeal temperature of 35-36oC. Continuous ultrafiltration is used to hemoconcentrate the perfusate, and modified ultrafiltration is used selectively. Low-dose inotropic support may facilitate separation from circulatory support. Fine-caliber lines for pressure monitoring and delivering infusions are placed into the atrium via the cannulation site and secured with a purse string. We do not routinely monitor the cavopulmonary pressure beyond the operative period. A single mediastinal chest tube is often adequate for drainage.
Postoperatively most patients are extubated within eight hours. Inotropic support is weaned over 24-48 hours followed by removal of intracardiac lines and the mediastinal tube. Frequently patients continue on digoxin and a diuretic through discharge.
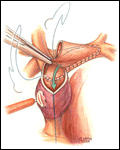
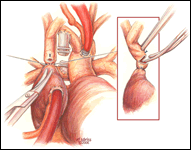
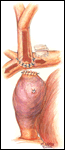
Alternatively, the bi-directional Glenn is another option to construct a superior cavopulmonary anastomosis (Figures 7-12). Somewhat simpler than the hemi-Fontan from a technical standpoint, a bi-directional Glenn does not address pulmonary artery hypoplasia or distortion by augmenting the pulmonary artery confluence, does not maintain the natural continuity of the right atrium and SVC to simplify a lateral tunnel Fontan completion, and does not enlarge the orifice between the SVC and the right atrium. For these reasons if the bi-directional Glenn is chosen as the cavopulmonary technique, the extracardiac conduit style Fontan is often the choice for the final stage of palliation.
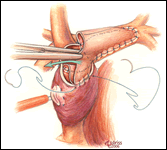
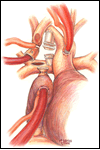
The preparation and conduct of cardiopulmonary bypass are the same as for the hemi-Fontan operation. Most often the bi-directional Glenn can be performed using continuous cardiopulmonary bypass, moderate hypothermia, and no cardioplegic arrest. First, the SVC to right atrium junction is over-sewn. The SVC is partially divided cephalad to this suture line. A venous cannula is then inserted into the open end of the SVC, connected to the circuit, and secured with a snare around the vessel. Once effective venous drainage of the superior systemic veins is accomplished, division of the SVC is completed keeping the vessel properly oriented to avoid twisting. Next, the right pulmonary artery is incised superiorly from a point medial to the upper lobe branch to the middle of the pulmonary artery confluence. Blood flow within the pulmonary artery can be occluded with a side-biting clamp or scavenged with cardiotomy suction. The SVC is connected to the right pulmonary artery in an end-to-side anastomosis using a running suture for the posterior line and interrupted sutures anteriorly to prevent a purse string effect and enable growth of the anastomosis. The venous cannula is clamped and withdrawn as the last sutures are tied. After completion of the anastomosis, gentle ventilation is commenced to facilitate flow of SVC blood through the lungs.
The patient is rewarmed to 37° C and weaned from cardiopulmonary bypass as described above. Postoperative care is similar to that outlined for patients who undergo a hemi-Fontan operation.
Tips & Pitfalls
- For the hemi-Fontan, the right atrial incision should be made in the superior-most portion of the chamber and extended carefully onto the medial side of the SVC to avoid disruption of the blood supply to the sinoatrial node.
- If antegrade pulmonary blood flow still exists at the time of the hemi-Fontan, the main pulmonary artery is transected and the pulmonary valve oversewn. Simple ligation of the main pulmonary artery may create a cul-de-sac beyond the pulmonary valve that serves as a cavity for stasis and thrombus formation, and therefore, is not recommended.
- The suture line between the homograft patch and the posterior wall of the right atrium caudad to the SVC-right atrial junction must be inspected carefully to assess for potential leak spots. Additional interrupted sutures may be necessary to avoid leaving a leak around the homograft dam and resultant systemic venous to pulmonary venous shunt.
- For construction of a bi-directional Glenn, marking sutures placed on the SVC anteriorly and laterally may assist in orienting the vessel to avoid twisting during creation of the anastomosis with the pulmonary artery.
- The anterior row of interrupted sutures placed in the bi-directional Glenn between the SVC and the pulmonary artery is helpful to reduce the chance of a purse string effect and subsequent stenosis. This technique is especially helpful for small caliber vessels.
Results
Table 1. Bi-Directional Cavopulmonary Connection and Hemi-Fontan, 1996-2004
| Author | # Patients | Type of Cavopulmonary Connection | Cardiopulmonary Bypass Strategy | Mean Age (months) | Mean Weight (Kg) | Hospital Survival % | Mean SaO2 (%) at Discharge | Morbidity |
|---|---|---|---|---|---|---|---|---|
| Douglas9 1999 |
114 | HF | DHCA | 5.4 | 6.1 | 98 | 80 | CHB 1.8% SND 5.3% PAT 4.4% |
| Aeba11 2000 |
35 | BCPC | CPB Moderate hypothermia |
14 | -- | 89 | 82 | PAT 2.9% |
| Jaquiss14 2004 |
85 | BCPC | CPB Moderate Hypothermia |
I. < 4 II. >4 | I. 4.8 II. 5.8 | I. 100 II. 100 | I. 79 II. 80 | -- |
| Jacobs5 1996 |
400 | HF | DHCA | 8.5 | -- | First 200 pts 92 Second 200 pts 96 |
-- | -- |
| Bradley7 1996 |
85 | 1) BCPC 2) BCPC with intraatrial patch 3) HF | CPB or DHCA | 4.8 | 5.6 | 94 | 80 | PAT 4% |
Legend: BCPC bidirectional cavopulmonary connection, CHB complete heart block, CPB cardiopulmonary bypass, DHCA deep hypothermic circulatory arrest, HF hemi-Fontan, PAT pulmonary artery thrombosis. SND sinus node dysfunction
References
- DeLeon SY, Idriss FS, Ilbawi MN, et al. The role of the Glenn shunt in patients undergoing the Fontan operation. J Thorac Cardiovasc Surg 1983;85:669-77.
- Bridges ND, Jonas RA, Mayer JE, Flanagan MF, Keane JF, Castaneda AR. Bidirectional cavopulmonary anastomosis as interim palliation for high-risk Fontan candidates. Early results. Circulation 1990;82(5 Suppl):IV-170-6.
- Donofrio MT, Jacobs ML, Norwood WI, Rychik J. Early changes in ventricular septal defect size and ventricular geometry in the single ventricle after volume-unloading surgery. J Am Coll Cardiol 1995;26:1008-15.
- Rychik J, Jacobs ML, Norwood WI. Acute changes in left ventricular geometry after volume reduction operation. Ann Thorac Surg 1995;60:1267-74.
- Jacobs ML, Rychik J, Rome JJ, et al. Early reduction of the volume work of the single ventricle: the hemi-Fontan operation. Ann Thorac Surg 1996;62:456-62.
- Slavik Z, Lamb RK, Webber SA, et al. Bidirectional superior cavopulmonary anastomosis: how young is too young? Heart 1996;75:78-82.
- Bradley SM, Mosca RS, Hennein HA, Crowley DC, Kulik TJ, Bove, EL. Bidirectional superior cavopulmonary connection in young infants. Circulation 1996;94(9 Suppl):II-5-11.
- Pizarro C, de Leval MR. Surgical variations and flow dynamics in cavopulmonary connections: a historical review. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 1998;1:53-60.
- Douglas WI, Goldberg CS, Mosca RS, Law IH, Bove EL. Hemi-Fontan procedure for hypoplastic left heart syndrome: outcome and suitability for Fontan. Ann Thorac Surg 1999;68:1361-8.
- Lardo AC, Webber SA, Friehs I, del Nido PJ, Cape EG. Fluid dynamic comparison of intra-atrial and extracardiac total cavopulmonary connections. J Thorac Cardiovasc Surg 1999;117:697-704.
- Aeba R, Katogi T, Kashima I, Omoto T, Kawada S, Omae K. Factors influencing arterial oxygenation early after bidirectional cavopulmonary shunt without additional sources of pulmonary blood flow. J Thorac Cardiovasc Surg 2000;120:589-95.
- Jacobs ML, Pourmoghadam KK. The hemi-Fontan operation. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2003;6:90-7.
- Bove EL, de Leval MR, Migliavacca F, Guadagni G, Dubini G. Computational fluid dynamics in the evaluation of hemodynamic performance of cavopulmonary connections after the Norwood procedure for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2003;126:1040-7.
- Jaquiss RDB, Ghanayem NS, Hoffman GM, et al. Early cavopulmonary anastomosis in very young infants after the Norwood procedure: impact on oxygenation, resource utilization, and mortality. J Thorac Cardiovasc Surg 2004;127:982-9.





Comments