ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Technique for Percutaneous Transfemoral Stent Graft Repair of Traumatic Aortic Transection
Patient Selection
Editor's Note
The technique proposed by Dr. Song provides an elegant solution to a problem of increasing magnitude : How do we stent a descending thoracic aorta which measures less than 24mm in diameter? The off-label use of abdominal aortic or peripheral vascular stents has certainly produced good results in the hands of the authors. However, given the fact that open techniques have yielded excellent results in young patients with normal caliber aortas and non-aneurysmal aortic pathology and since availability of smaller diameter thoracic aortic stents with FDA approval is only a matter of time, we would recommend extreme caution before adapting the technique presented here.
Traumatic thoracic aortic transection (TSXN) is a potentially lethal injury that occurs following blunt injury to the chest, most commonly as the result of motor vehicle crashes or falls [1]. Endograft repair of thoracic aortic TSXN is increasingly utilized as a less invasive alternative to open surgical repair [2]. Currently available thoracic endografts, the smallest of which is 26 mm in diameter, are designed for use in older adults with enlarged aortas. The average thoracic aortic diameter of young adults is less than 20 mm [3-5]. Collapse of excessively oversized stent grafts when used to treat traumatic aortic TSXN in small diameter aortas has been reported, sometimes with catastrophic results [6,7]. Short (less than 4 cm in length), small-diameter proximal aortic endograft cuffs from abdominal systems have been used for treatment of thoracic aortic TSXN [7,8]. The delivery catheters of currently available abdominal devices are frequently inadequate in length to reach the thoracic aortic isthmus. This deficiency can require direct surgical access to the iliac arteries in order for the device to reach the site of injury [8,9]. Here, we describe a technique for percutaneous transfemoral treatment of aortic TSXN in patients with small diameter thoracic aortas using commercially available large iliac limb extender endografts (ESLE 20 through 24-55, Zenith, Cook Group Inc, Bloomington, IN) delivered through long 16 or 18 French sheaths.
The diagnosis of thoracic aortic TSXN is typically made by contrast-enhanced CT scan shortly after arrival in the emergency department. Axial images from this study as well as multi-planar reformats are used to define the extent of the injury and confirm the appropriateness of stent graft repair. Imaging from the head to the common femoral arteries is reviewed. Adequate landing zones and femoral artery access are confirmed on this study. Aberrant arch anatomy that would preclude stent graft repair, such as aberrant right subclavian artery, is excluded. In our experience, the diameter of the normal aorta in young adults proximal to the tear has been in the 19 mm range. The diameter of the normal aorta distal to the tear has been in the 16 mm range. Aortas in this size range are not suitable for repair with currently available thoracic endografts and should be considered for the procedure described here.
Operative Steps
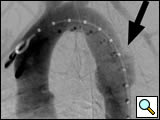 |
| Figure 1: Left anterior oblique aortogram obtained with measuring pigtail catheter demonstrating aortic arch anatomy and location of aortic transection. |
The patients are usually intubated as part of their initial resuscitation and have general anesthesia provided by an anesthesiology team for the procedure. In our institution, the procedure is performed in a conventional angiographic suite using ceiling-mounted Siemens Axiom ARTIS equipment (Erlangen, Germany). Patients are positioned supine with both groins prepped and draped. A 5-F graduated measuring pigtail catheter (Angiodynamics, Queensbury, NY) is inserted percutaneously via the left common femoral artery and a left anterior oblique arch aortogram is obtained to confirm arch anatomy and the location of the injury (Figure 1). Additional views, including selective vertebral artery injections, are performed as necessary.
The measuring pigtail catheter is also used to determine the length from the femoral artery access to the arch. Abdominal and iliac endografts typically come preloaded on delivery catheters that are 55 cm long. This length is insufficient for delivery from the femoral artery to the proximal descending thoracic aorta in most adults. The endograft delivery system must therefore be modified to permit this repair via a percutaneous transfemoral approach.
The next step is to prepare the Zenith iliac extender endograft for delivery through a longer sheath. The Zenith iliac limb extension is a self-expanding endograft constructed from 3 stainless-steel Z-stents sewn inside a 5.5 cm long tubular Dacron graft (Figure 2A). The limbs are supplied preloaded in 55 cm-long sheaths on either 16- or 18-F delivery catheters. The delivery catheter is constructed with a central metal hypotube that accommodates a 0.035-inch guidewire. The hypotube is imbedded in the delivery catheter plastic, with a gap in the catheter material near the distal end to accommodate the compressed endograft in the loaded, sheathed state (Figure 2B). The distal portion of the delivery catheter is a 10 cm long tapered tip (Figure 2C). This tip is permanently attached to the hypotube. The usual mechanism of deployment of the iliac limb is to withdraw the outer sheath while maintaining the inner delivery catheter in stable position. The endograft opens as each Z-stent is exposed by the sheath withdrawal, but is held in position by the delivery catheter until the final stent deploys. Once the endograft is completely opened, the delivery catheter can be withdrawn through endograft.
Our approach is to transfer the endograft from the short sheath in which it is packaged to a long sheath over a guidewire, without deploying it. The first step is to cut off the tapered tip of the delivery catheter. It is essential to avoid crimping the central hypotube during the amputation so that the endograft transfer can be performed over a guidewire. After flushing the delivery sheath according to the manufacturer’s instructions, the delivery catheter is advanced just enough so that the bare hypotube proximal to the tapered tip is exposed beyond the end of the sheath, but the endograft remains within the sheath (Figure 2D). Leaving the central shipping stylet within the hypotube, an oscillating sterile surgical saw (Stryker, Kalamazoo, MI) is used to amputate the dilator tip. The stylet is then removed and the hypotube lumen tested to accommodate a 0.035-inch guidewire.
Long sheaths are then selected for deployment of the endograft. A 16 French 80 cm sheath is used for 20 mm diameter endografts, and 18 French 80 cm sheaths are used for 22 and 24 mm diameter devices. Custom sheaths with extra-large check-flow valves were used in our initial cases. Currently we use non-custom 18 French Keller-Timmerman sheaths (Cook Group, Inc). A curve is steamed in the distal end of each sheath that approximates the aortic arch. The sheaths are then flushed.
Percutaneous access is then obtained via the right common femoral artery. A Perclose Proglide device (Abbott Vascular, Redwood, CA) is used to pre-deploy a suture in the common femoral artery [10]. Using a floppy-tipped guidewire a 100 cm angled catheter is advanced into the ascending aorta and the wire is exchanged for a 260 cm 0.035 Amplatz superstiff guidewire (Boston Scientific Inc, Natick, MA). Using serial dilatation over the guidewire, a 16 or 18-French 80 cm sheath as described above is introduced into the aortic arch.
The modified original delivery sheath is then inserted snuggly into the hub of the long 16- or 18-French sheath over the guidewire. The endograft is transferred into the new sheath by advancing the delivery catheter while ensuring that the end of the original sheath is tightly nested in the receiving sheath. The original delivery system is then removed over the guidewire. The blunt back end of the dilator from the new sheath is inserted over the guidewire and used to push the endograft to the end of the long delivery sheath. The 5-French pigtail catheter is re-advanced into the distal aortic arch and control angiograms are obtained through the 5-French pigtail catheter prior to deployment of the endograft (Figure 3A). The endograft is deployed by withdrawing the sheath while maintaining the endograft in a fixed position with the pusher catheter. Angiograms are obtained through the 5 French pigtail catheter before and after gentle inflation of appropriate sized angioplasty balloons within the endografts (Figure 3B).
The large sheath is removed and hemostasis obtained by securing the Perclose sutures. Manual compression is used for hemostasis after removal of the pigtail catheter from the left common femoral artery.
Tips & Pitfalls
The treatment of thoracic aortic TSXN with endografts presents several technical challenges. The most important is the lack of a dedicated device for these patients. All commercially available aortic endografts, especially those for use in the thoracic aorta, were designed for treatment of aneurysms and dissections. These pathologies usually occur in older individuals with intrinsic arterial disease and larger diameter aortas. The smallest commercially available thoracic aortic endograft, the Gore TAG device (W.L. Gore & Associates, Flagstaff, AZ) is 26 mm diameter, and is not recommended for use in aortas less than 23 mm in diameter [12]. One concern with excessive endograft oversizing is in-folding of the graft material that prevents an adequate seal against the aortic intima. Of greater concern, graft collapse has been reported when this device is used in aortas smaller than 23 mm diameter to treat an aortic TSXN [2,6]. The mechanism of graft collapse is unclear but this complication has also been reported in the treatment of dissection with Gore TAG and with other thoracic endografts [13,14].
The size of the delivery system of the Gore TAG device presents another challenge in the application to young trauma patients. A 20-French sheath (with an almost 7.6 mm outer diameter) is required to introduce the 26 mm diameter endograft. The average external iliac artery diameter in our experience has been only 6 mm. In order to use the Gore device in patients with small external iliac arteries, retroperitoneal exposure of the common iliac artery or distal abdominal aorta may be required to find a vessel that can accommodate a large introducer sheath [15].
As a result of the lack of a dedicated device for thoracic aortic TSXN many operators employ endograft components, usually proximal cuffs from abdominal aortic endograft systems, to treat this injury. These cuffs are short in length and pre-mounted on delivery systems that are intended for use in the abdominal aorta. Due to the short length of the endografts, multiple overlapping devices are usually needed to adequately exclude an aortic TSXN [2,8,9,15]. The delivery systems are often not of adequate length to reach the proximal thoracic aorta, necessitating surgical exposure of the external or common iliac artery, or even the distal abdominal aorta [9].
Zenith iliac limbs are of more appropriate length (5.5 cm) for use in the thoracic aorta. A second advantage of our technique is the ability to use a transfemoral percutaneous approach independent of the length of the factory-supplied endograft delivery catheter. A key step in the procedure is the transfer to the iliac limb from the shorter (55 cm) factory-supplied sheath to the longer (80 cm) delivery sheath. Amputation of the 10 cm long grey dilator tip using a sterile saw to cut the hypotube with the stylet in place ensures that the iliac limb can be transferred over a guide wire from one sheath to another. Long delivery sheaths with either an extra-large check-flow valve or a clampable valve are essential to accept the end of the 16 or 18-F Zenith delivery sheath.
The ability to treat the aortic TSXN through an 18 French or smaller sheath is desirable in young adults who may have small femoral and external iliac arteries [15]. This advantage is not unique to the Zenith iliac limbs, as the Gore Excluder (18-F sheath) and Medtronic (21 French outer diameter catheter) proximal abdominal cuffs have smaller delivery systems than the smallest Gore TAG (20-F sheath).
The long term behavior of the Zenith iliac limb extender endograft in the thoracic aorta is unknown. Patients who survive repair of traumatic aortic injury will likely live many decades. The optimal follow-up regimen including appropriate imaging to assess graft integrity, graft stability, remains in evolution. Patient selection for this type of repair must take into consideration these long-term uncertainties in addition to the immediate condition of the patients. Careful follow-up will be essential to elucidate the extended outcomes of endograft repair of traumatic thoracic aortic TSXN with any device.
Recently, an extended Zenith proximal abdominal aortic cuff (ESBE) has become available that has diameters similar to the iliac extenders and is 58 mm in length. The delivery systems are the same French size as the analogous iliac limbs, but are only 40 cm in length. We have not yet utilized one of these devices to treat a patient with TSXN, but anticipate employing the same transfer technique as used for the iliac limbs.
A dedicated endograft for treatment of patients with traumatic TSXN is needed. Ideally, such a device would be 60-70 mm in length, with diameters of 20, 22 and 24 mm, have a low profile for insertion through the small pelvic arteries found in young patients, and be adapted for the sharp angulation of the normal distal aortic arch. Additionally, dissolvable or retrievable devices would be appealing in this patient population who have a short term acute need for control of their aortic injury. Until such devices become available, adaptation of currently available endografts intended for placement in the abdominal aorta or iliac arteries will be necessary for endovascular treatment of patients with small aortas.
References
- Nzewi O, Slight RD, Zamvar V. Management of blunt thoracic aortic injury. Eur J Vasc Endovasc Surg 2006;31:18-27
- Tehrani HY, Peterson BG, Katariya K, et al. Endovascular repair of thoracic aortic tears. Ann Thorac Surg 2006;82:873-877;discussion 877-878.
- Garcier JM, Petitcolin V, Filaire M, et al. Normal diameter of the thoracic aorta in adults: a magnetic resonance imaging study. Surg Radiol Anat 2003;25:322-329.
- Poutanen T, Tikanoja T, Sairanen H, Jokinen E. Normal aortic dimensions and flow in 168 children and young adults. Clin Physiol Funct Imaging 2003;23:224-229.
- Trivedi KR, Pinzon JL, McCrindle BW, Burrows PE, Freedom RM, Benson LN. Cineangiographic aortic dimensions in normal children. Cardiol Young 2002;12:339-344.
- Idu MM, Reekers JA, Balm R, Ponsen KJ, de Mol BA, Legemate DA. Collapse of a stent-graft following treatment of a traumatic thoracic aortic rupture. J Endovasc Ther 2005;12:503-507.
- Neschis DG, Moaine S, Gutta R, et al. Twenty consecutive cases of endograft repair of traumatic aortic disruption: lessons learned. J Vasc Surg 2007;45:487-492.
- Peterson BG, Matsumura JS, Morasch MD, West MA, Eskandari MK. Percutaneous endovascular repair of blunt thoracic aortic transection. J Trauma 2005;59:1062-1065.
- McPhee JT, Asham EH, Rohrer MJ, et al. The midterm results of stent graft treatment of thoracic aortic injuries. J Surg Res 2007;138:181-188.
- Watelet J, Gallot JC, Thomas P, Douvrin F, Plissonnier D. Percutaneous repair of aortic aneurysms: a prospective study of suture-mediated closure devices. Eur J Vasc Endovasc Surg 2006;32:261-265.
- Hager A, Kaemmerer H, Rapp-Bernhardt U, et al. Diameters of the thoracic aorta throughout life as measured with helical computed tomography. J Thorac Cardiovasc Surg 2002;123:1060-1066.
- Gore WL. Instructions for use for: Gore Thoracic Endoprosthesis. In: WL Gore and Associates, 2005.
- Steinbauer MG, Stehr A, Pfister K, et al. Endovascular repair of proximal endograft collapse after treatment for thoracic aortic disease. J Vasc Surg 2006;43:609-612.
- Melissano G, Tshomba Y, Civilini E, Chiesa R. Disappointing results with a new commercially available thoracic endograft. J Vasc Surg 2004;39:124-130.
- Milas ZL, Milner R, Chaikoff E, Wulkan M, Ricketts R. Endograft stenting in the adolescent population for traumatic aortic injuries. J Pediatr Surg 2006;41:e27-30.
- Saad NE, Pegoli W, Alfieris G, Waldman DL, Davies MG. Endovascular repair of a traumatic aortic transection in a pediatric patient [In Process Citation]. J Vasc Interv Radiol 2007;18:443-446.










