ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Adjunctive Surgical Atrial Fibrillation Ablation during Cardiac Surgery: Real Life Experiences
Introduction
Atrial fibrillation (AF) is a common rhythm disorder that is responsible for significant morbidity and mortality due to thromboembolism, stroke, and heart failure, in addition to adverse reactions secondary to the use of antiarrhythmic drugs (1, 2, 3). Valvular heart diseases, especially of the mitral valve, cause structural cardiac changes, atrial remodeling, and increase the likelihood of atrial dysrhythmia (4). The prevalence of atrial fibrillation in individuals undergoing cardiac surgery ranges between 1-9% in isolated coronary artery bypass grafting procedures, and up to 50% in patients in need of mitral valve surgery (5).
The first effective surgical treatment for AF was introduced in 1987 by Dr. James Cox. The Cox-Maze (CM) I procedure is a cut-and-sew technique, designed to create lines of scar tissue in order to terminate the macro re-entry circuits in the atria, thereby preventing the atrium from fibrillating (6). Due to the high incidence of postoperative complications, the original CM procedure was modified twice and resulted in the Cox-Maze III procedure. This technique became the gold standard for surgical treatment of AF (7). In order to simplify the Maze operation further, Melby and colleagues developed the Cox-Maze IV procedure (8). This technique used radiofrequency energy or cryo-energy to create lesions with comparable efficacy to the CM-III technique (9).
In this study, the authors sought to assess the efficacy of radiofrequency and cryo-ablation during typical cardiac surgeries using prolonged (4-7 days) ambulatory Holter monitoring. Eight patients with recurrent AF underwent an electrophysiological study with a complete electro-anatomical reconstruction of the left atrium.
Methods
Study Patients
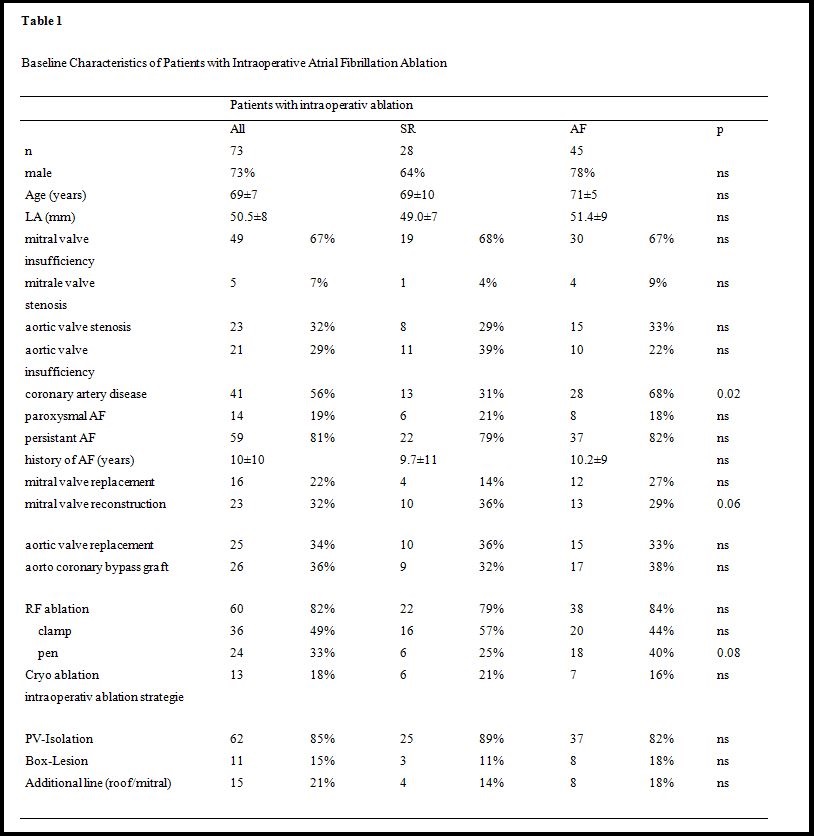
The study consisted of 73 consecutive patients who underwent left atrial ablation during cardiac surgery for mitral and aortic valve disease, coronary artery disease, or any combination of these, with evidence of persistent (n=59, 81%) or paroxysmal AF (n=14, 19%, Table 1). All of these patients were symptomatic and had a history of AF for 10±10 years. 49 patients (67%) were on Amiodarone.
Ablation During Cardiac Surgery
Intraoperative ablation was performed using an argon cryoprobe (Surgifrost TM, CryoCath Technologies, Medtronic Inc., USA), which can be shaped to the left atrial anatomy, creating linear lesions between 10 and 60 mm, and RF energy with a monopolar saline-irrigated RF ablation catheter (Cardioblate, Medtronic Inc., Minneapolis, USA), or a bipolar ablation clamp (Cardioblate BP22, Medtronic Inc., Minneapolis, USA). The temperature setting on the cryoprobe was144 °C and the application time was 45 seconds. The setting for each application of RF-energy was 30 Watts for 30 seconds. The ablation approach included ipsilateral PV-isolation or a box lesion, a posterior linear lesion connecting the ipsilateral PVs at the roof of the LA, and a lesion connecting the lateral PVs to the mitral annulus. Lesion completeness was only checked visually or assumed when impedance readout dropped during RF-application. A test for completeness of lesion, such as pacing or activation map, was not performed.
Management and Follow-Up After Intraoperative Ablation
All 73 patients were monitored with continuous ECG recording for two post-operative days. Antiarrhythmic drug regimens with Amiodarone were continued or initiated if patients had recurrent atrial arrhythmias during hospitalization. Electrical cardioversion was performed if necessary.
Patients were seen in 3-6 month intervals at the University Heart Center arrhythmia outpatient clinic. A 4-7 day Holter was performed at each visit, or if arrhythmias were noted. A recurrent arrhythmia was defined as any atrial arrhythmia longer than 30 seconds, irrespective of symptoms and without any blanking period. Patients with symptomatic recurrence, which could not be controlled with antiarrhythmic drugs, were offered a catheter ablation.
Electrophysiological Approach
Patients with symptomatic recurrent AF or atrial tachycardia during the follow-up period underwent an electrophysiological study using an electro-anatomical reconstruction of the left atrium (10). In short, two standard catheters (6 French Biosense-Webster, Diamond Bar, USA) were positioned at the His-bundle region via a femoral vein and in the coronary sinus via the left subclavian vein. Two SL-1 sheaths (8 and 8.5 French, St. Jude Medical, St. Paul, USA) were advanced to the left atrium using a modified Brockenbrough technique. The method of 3-dimensional electro-anatomical mapping in the left atrium has been previously described in detail (11). Mapping was performed with a 3.5 mm-tip catheter (ThermoCool Navi-Star, Biosense-Webster, Diamond Bar, USA) during coronary sinus pacing, sinus rhythm, atrial macroreentry tachycardia, or atrial fibrillation. Each PV ostium was identified by selective venography and tagged on the electro-anatomical map. A single decapolar lasso catheter (Biosense-Webster, Diamond Bar, USA) was placed within each vein and left in the superior PVs during radiofrequency (RF) application. Irrigated RF energy was delivered with a target temperature of 43°C, a maximum power limit of 40 W, and an infusion rate of 17 mL/min. The power was set to a maximum of 25 Watts in the vicinity of the esophagus, equipped with a temperature probe (Sensitherm, SJM, St. Paul, USA). RF energy was applied for at least 30 seconds or until the peak amplitude of the local electrogram amplitude had decreased by about 70%, or double potentials were noted. Irrigated RF ablation was performed in the posterior wall more than 1 cm and in the anterior wall more than 5 mm from the angiographically-defined PV ostium. The endpoint of the circumferential ablation was defined as absence of any PV spike documented within the ipsilateral PVs. Intra-atrial ablation lines were verified during sinus rhythm or CS pacing with double potentials and a shift of activation during completion of the line.
Management After EPE and Catheter Ablation
Patients were monitored for 24 hours after all ablation procedures. Intravenous Heparin was administered for two days, followed by Warfarin for at least three months. Long term anticoagulation was used according to CHA2DS2Vasc-score (congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke (doubled), vascular disease, age 65-74, sex (female sex)). All patients were kept on previous antiarrhythmic drugs for three months after ablation.
Patients were followed for 9±5 months in regular intervals at the arrhythmia outpatient clinic at the University Heart Centre. A 4-7 day Holter was performed at each visit. A recurrent arrhythmia was defined as any atrial arrhythmia longer than 30 seconds irrespective of symptoms.
Statistical Considerations
All data were recorded and evaluated with SPSS statistical software version 11.5. All continuous variables are presented as means ± standard deviation (SD) or as numbers and percentages, as appropriate. Categorical variables were presented as proportions. The identified variables and all procedural data with abnormal distribution were compared using a Kruskal-Wallis test for significance between the ablation groups. If significant, the Mann Whitney-U-Test was used to compare the individual groups. A two-sided probability value < 0.05 was considered to be statistically significant.
Results
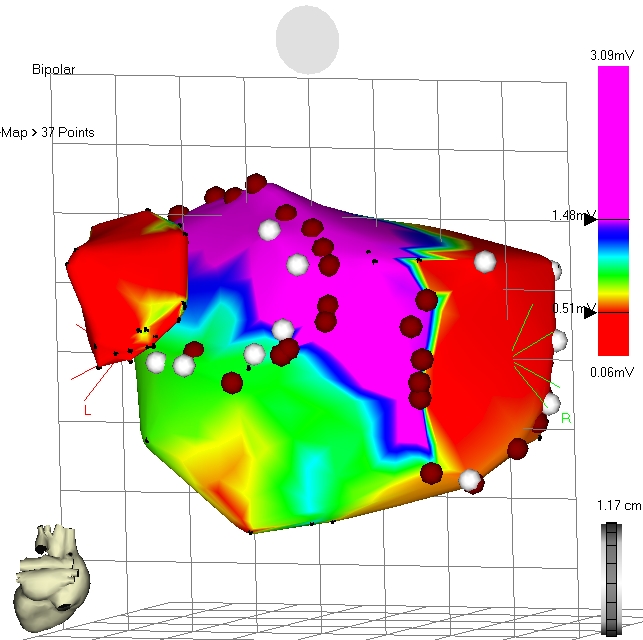
Figure 2: Electro-anatomical map of the left atrium during sinus rhythm.
Operative Course
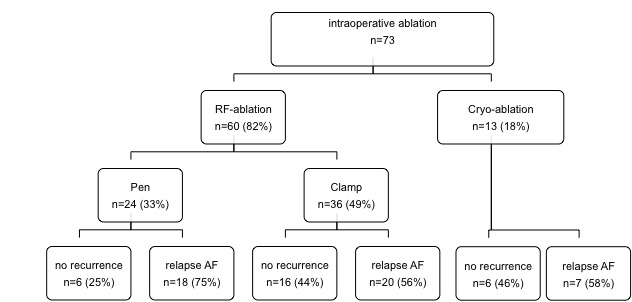
60 (82%) of 73 patients with surgery for mitral valve (53%), aortic valve (34%), and coronary artery disease (36%) - or a combination of these - underwent intraoperative ablation with RF energy and 13 patients (18%) with cryo (Table 1). RF-ablation was performed with a pen in 24 (33%) and by use of a clamp in 36 patients (49%). In all patients PV were isolated, in 11 cases (15%) using a box lesion and 15 patients (21%) received an additional roof- and mitral-isthmus line (Table 1).
Arrhythmias during Follow-Up After Intraoperative Ablation
During a mean follow-up of 23±11 months, 6 patients (25%) survived free of AF after intraoperative ablation with a pen, 16 patients (57%) had no recurrence after PV isolation with a clamp, and 6 patients (21%) survived free of AF after cryo-ablation (p=0.08, Table 1). Mitral valve reconstruction (as opposed to mitral valve replacement, p=0.06) and the absence of coronary disease were associated with improved AF free survival (p=0.02). Patients’ characteristics including LVEF, NYHA class, age, duration of AF, or interventional characteristics such as additional lines or box lesions had no significant impact on arrhythmia recurrence (Table 1).
Electrophysiological Work-Up
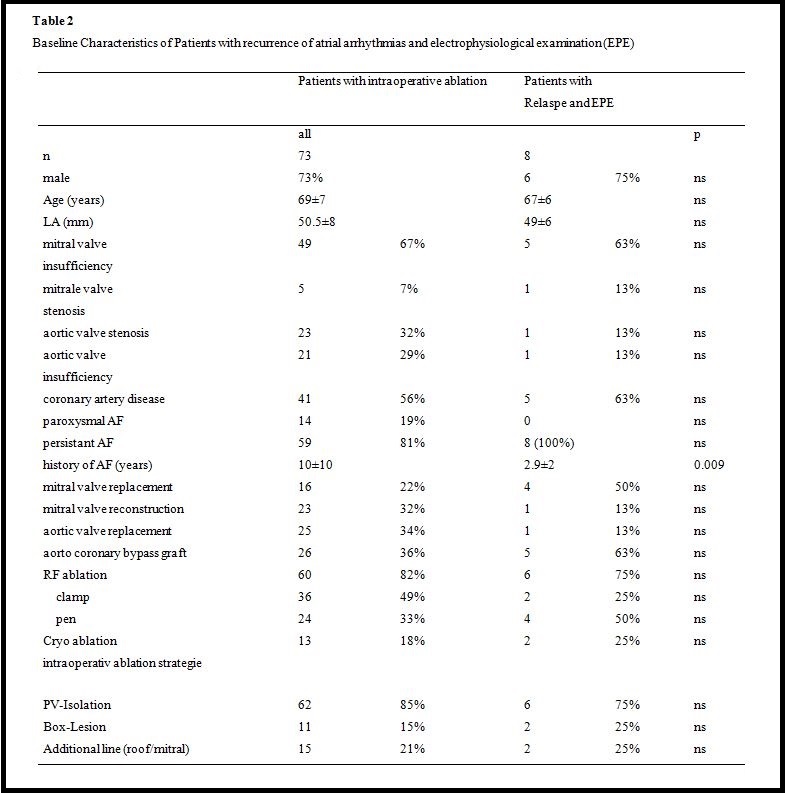
Eight (18%) of 45 patients with relapse of AF (four (50%) after intraoperative pen ablation, two (25%) after clamp ablation, and two (25%) after cryo-ablation) underwent an electrophysiological study with electro-anatomical reconstruction of the left atrium (Table 2). In one patient (13%), an additional right atrial incisional tachycardia was mapped and ablated. Seven patients (88%) were in persistent AF, and one patient had both a left atrial macro-reentry tachycardia and a right atrial incisional tachycardia.
Septal circumferential lesions were demonstrated during mapping with discrete gaps, mostly inferior anterior and superior posterior (Figure 2). All lateral veins were isolated (anterior close the ostium, posteriorly deep inside the vein) leaving the antral untreated. Neither roof lines nor mitral isthmus lines showed bidirectional conduction block. In one case, all lines were blocked when the lateral veins were isolated at the antral side (Figure 3). A separate induced incisional right atrial tachycardia was ablated at the free wall of the right atrium in one patient.
Follow-Up After Catheter Ablation
The eight symptomatic patients (18%) with relapse of AF and subsequent catheter ablation were followed up for 9±5 months, seven (87%) were free of any atrial arrhythmia without taking antiarrhythmics. The eighth patient had paroxysmal AF and underwent a second electrophysiological catheter ablation. He showed a gap in the line around the lateral veins, which was eventually completed.
Discussion
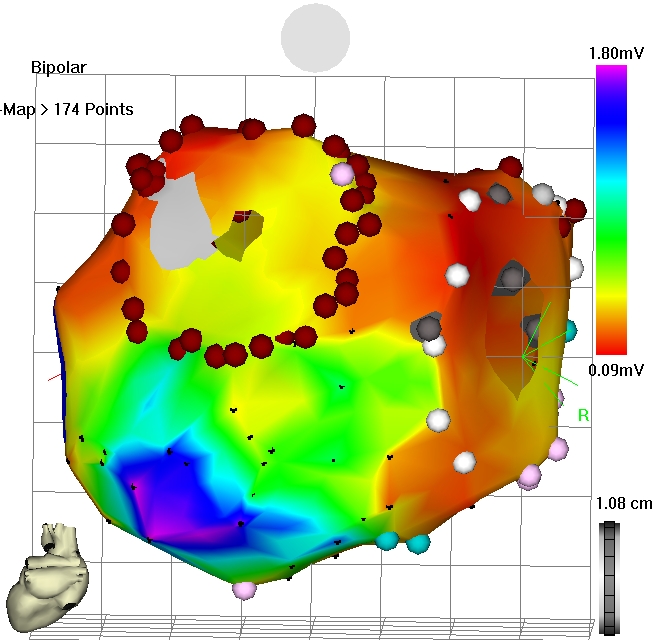
Figure 3: Electro-anatomical voltage map after cryo-ablation during sinus rhythm.
The examination showed sobering results of AF ablation with using intraoperative tools during cardiac surgery. Only 47% of all patients were free of AF after clamp ablation, 46% after cryo-ablation, and 25% after RF-ablation with a pen. These results are worse than previously reported (75.9% AF free survival after three years) in patients with a much shorter history of AF (12 – 14). The authors’ results are closer to those reported by Iwamura after maze in mitral and non-mitral valve surgery with 57% to 61% stable sinus rhythm after two years (15). The results are also closer to the data from Wang et al., who included patients with a history of AF of 61 to 81 months and found 62 % of patients were in SR without taking antiarrhythmic drugs after two years (16, 17). The sobering results may be due to the inclusion of very long persistent AF (1010 years), and the uncontrolled intraoperative methodology.
Eventually, electro-anatomical mapping in those patients with recurrent persistent AF revealed incomplete ablation lines or too distal ablation in the lateral veins. All septal veins demonstrated gaps and the lateral veins were isolated at a level to distal in the vein, leaving the antrum untreated. Recent insight from a new strategy supports this view. A “cut-and-sew” strategy will render continuous lines of block, and revealed significantly better AF free survival than surgical ablation (18). Therefore, the authors believe that the unsatisfactory results from uncontrolled isolation of veins lack control by conduction testing. However, the surgical approach turned out to set the stage for an easy electrophysiological finish with completion of ablation lines and promising short-term results in patients who could not be treated by interventional electrophysiologists because of the underlying heart disease. All eight patients with catheter ablation were in sinus rhythm without antiarrhythmic drugs, and only one patient required a re-ablation of the lateral vein. The electrophysiological work-up may also impact the surgical approach to AF ablation and help improve the intraoperative ablation tools based on insights from electro-anatomical mapping.
Limitations
Patients were unselected as far as structural heart disease and duration of AF were concerned. Moreover surgical ablation techniques were inconsistent. However, the heterogenic approach may in fact mirror the uncontrolled use of ablation devices in real life.
Conclusion
Intraoperative AF ablation during cardiac surgery may render sobering results in unselected patients. The results may be improved with an electrophysiological endpoint during the procedure, a more antral ablation of the lateral veins, and better selection of patients. The surgical approach turned out to set the stage for an easy electrophysiological finish with completion of ablation lines and promising short-term results in patients who could not otherwise be treated by interventional electrophysiologists.
References
- V. Fuster, L. E. Ryden, D. S. Cannom, H. J. Crijns, A. B. Curtis, K. A. Ellenbogen et al. Acc/aha/esc 2006 guidelines for the management of patients with atrial fibrillation: A report of the american college of cardiology/american heart association task force on practice guidelines and the european society of cardiology committee for practice guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation): Developed in collaboration with the european heart rhythm association and the heart rhythm society. Circulation 2006;114:e257-354.
- P. Santangeli, L. Di Biase and A. Natale. Ablation versus drugs: What is the best first-line therapy for paroxysmal atrial fibrillation? Antiarrhythmic drugs are outmoded and catheter ablation should be the first-line option for all patients with paroxysmal atrial fibrillation: Pro. Circulation Arrhythmia and electrophysiology 2014;7:739-46.
- G. Y. Lip, H. F. Tse and D. A. Lane. Atrial fibrillation. Lancet 2012;379:648-61.
- A. Harada, K. Sasaki, T. Fukushima, M. Ikeshita, T. Asano, S. Yamauchi et al. Atrial activation during chronic atrial fibrillation in patients with isolated mitral valve disease. The Annals of thoracic surgery 1996;61:104-11; discussion 11-2.
- I. Romero, S. Nedios and C. Kriatselis. Diagnosis and management of atrial fibrillation: An overview. Cardiovascular therapeutics 2014;32:242-52.
- J. L. Cox, R. B. Schuessler, H. J. D'Agostino, Jr., C. M. Stone, B. C. Chang, M. E. Cain et al. The surgical treatment of atrial fibrillation. Iii. Development of a definitive surgical procedure. The Journal of thoracic and cardiovascular surgery 1991;101:569-83.
- H. Calkins, K. H. Kuck, R. Cappato, J. Brugada, A. J. Camm, S. A. Chen et al. 2012 hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart rhythm: the official journal of the Heart Rhythm Society 2012;9:632-96 e21.
- S. J. Melby, A. Zierer, M. S. Bailey, J. L. Cox, J. S. Lawton, N. Munfakh et al. A new era in the surgical treatment of atrial fibrillation: The impact of ablation technology and lesion set on procedural efficacy. Annals of surgery 2006;244:583-92.
- B. Chiappini, S. Martin-Suarez, A. LoForte, G. Arpesella, R. Di Bartolomeo and G. Marinelli. Cox/maze iii operation versus radiofrequency ablation for the surgical treatment of atrial fibrillation: A comparative study. The Annals of thoracic surgery 2004;77:87-92.
- F. Ouyang, D. Bansch, S. Ernst, A. Schaumann, H. Hachiya, M. Chen et al. Complete isolation of left atrium surrounding the pulmonary veins: New insights from the double-lasso technique in paroxysmal atrial fibrillation. Circulation 2004;110:2090-6.
- S. Ernst, T. Broemel, U. Krumsdorf, H. Hachiya, F. Ouyang, C. Linder et al. [three-dimensional reconstruction of pulmonary veins and left atrium. Implications for catheter ablation of atrial fibrillation]. Herz 2003;28:559-65.
- S. Maltais, J. Forcillo, D. Bouchard, M. Carrier, R. Cartier, P. Demers et al. Long-term results following concomitant radiofrequency modified maze ablation for atrial fibrillation. Journal of cardiac surgery 2010;25:608-13.
- S. Geidel, K. Krause, S. Boczor, K. H. Kuck, M. Lass, J. Ostermeyer et al. Ablation surgery in patients with persistent atrial fibrillation: An 8-year clinical experience. The Journal of thoracic and cardiovascular surgery 2011;141:377-82.
- S. Geidel, M. Lass, K. Krause, C. Schneider, S. Boczor, K. H. Kuck et al. Persistent atrial fibrillation ablation concomitant to coronary surgery. The Thoracic and cardiovascular surgeon 2011;59:207-12.
- T. Iwamura, K. Kajimoto, T. Yamamoto, M. Yamasaki, K. Tambara, Y. Yoneda et al. Mid-term results for the maze procedure in patients with non-mitral valvular atrial fibrillation. Annals of thoracic and cardiovascular surgery : official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia 2011;17:356-62.
- J. Wang, Y. Li, J. Shi, J. Han, C. Xu, C. Ma et al. Minimally invasive surgical versus catheter ablation for the long-lasting persistent atrial fibrillation. PloS one 2011;6:e22122.
- J. G. Wang, Y. Li, J. H. Shi, J. Han, Y. Q. Cui, T. G. Luo et al. Treatment of long-lasting persistent atrial fibrillation using minimally invasive surgery combined with irbesartan. The Annals of thoracic surgery 2011;91:1183-9.
- L. M. Pires, T. L. Leiria, G. G. de Lima, M. L. Kruse, I. A. Nesralla and R. A. Kalil. Comparison of surgical cut and sew versus radiofrequency pulmonary veins isolation for chronic permanent atrial fibrillation: A randomized study. Pacing Clin Electrophysiol 2010;33:1249-57.




Comments